Abstract
The use of a nonlethal selection scheme, most often using the aadA gene that confers resistance to spectinomycin and streptomycin, has been considered critical for recovery of plastid transformation events. In this study, the plastid-lethal markers, glyphosate or phosphinothricin herbicides, were used to develop a selection scheme for plastids that circumvents the need for integration of an antibiotic resistance marker. The effect of selective agents on tobacco (Nicotiana tabacum) mesophyll chloroplasts was first examined by transmission electron microscopy. We found that at concentrations typically used for selection of nuclear transformants, herbicides caused rapid disintegration of plastid membranes, whereas antibiotics had no apparent effect. To overcome this apparent herbicide lethality to plastids, a “transformation segregation” scheme was developed that used two independent transformation vectors for a cotransformation approach and two different selective agents in a phased selection scheme. One transformation vector carried an antibiotic resistance (aadA) marker used for early nonlethal selection, and the other transformation vector carried the herbicide (CP4 or bar) resistance marker for use in a subsequent lethal selection phase. Because the two markers were carried on separate plasmids and were targeted to different locations on the plastid genome, we reasoned that segregation of the two markers in some transplastomic lines could occur. We report here a plastid cotransformation frequency of 50% to 64%, with a high frequency (20%) of these giving rise to transformation segregants containing exclusively the initially nonselected herbicide resistance marker. Our studies indicate a high degree of persistence of unselected transforming DNA, providing useful insights into plastid chromosome dynamics.
Since the first report of tobacco (Nicotiana tabacum) plastid transformation (Svab et al., 1990), the use of the aadA gene to confer spectinomycin/streptomycin resistance has been by far the most reliable and reproducible selectable marker for production of plastid transformants in tobacco (Golds et al., 1993; O'Neill et al., 1993; Svab and Maliga, 1993) and in other species such as potato (Solanum tuberosum; Sidorov et al., 1999), Arabidopsis (Sikdar et al., 1998), rice (Oryza sativa; Khan and Maliga, 1999), and tomato (Lycopersicon esculentum; Ruf et al., 2001), even though a few alternative selectable markers have also been reported (Carrer et al., 1993; Daniell et al., 2001). Although aadA selection allows for efficient and reproducible plastid transformation in numerous plant species, the antibiotic resistance gene is not needed in the final transgenic product and its removal is desirable to eliminate the theoretical risk of antibiotic resistance gene flow. Methods used to remove the antibiotic resistance marker from transplastomic plants have included marker excision using the Cre/lox site-specific recombination system (Corneille et al., 2001; Hajdukiewicz et al., 2001) and generation of deletion derivatives after homologous recombination-mediated excision (Fischer et al., 1996; Iamtham and Day, 2000). Although marker excision using these methods has been achieved, they are complicated by the need to develop additional transgenic plant lines and can give alternative excision products, making it more difficult to identify desired lines.
Herbicide resistance markers provide attractive alternatives to antibiotic resistance markers and have been widely used in nuclear transformation of many plant species (Zhou et al., 1995; Mannerlof et al., 1997; Clemente et al., 2000). However, antibiotic resistance using aadA in plastids has been considered a nonlethal selection scheme, critical for recovery of plastid transformants (Maliga et al., 1993), whereas herbicide resistance using CP4 or bar is generally considered a lethal selection scheme in plants (De Block et al., 1989; Barry et al., 1992; Cao et al., 1992; Padgette et al., 1996). For this reason, use of herbicide resistance markers has not yet been extended to plastid transformation. In nuclear transformation, insertion of the transgene into the nuclear genome and subsequent expression of the selectable marker provides protection for the entire cell. On the other hand, in plastid transformation, presumably only one plastid is transformed, with only one or a few plastid genomes initially incorporating the transgene. Therefore, the lethal selection scheme kills the cells at an early stage of transformation before the integrated plastid transgene has had time to amplify sufficiently to express phenotypic resistance at the cellular level.
Whereas antibiotics prevent plastid protein synthesis by binding to 70S ribosomal subunits, herbicides affect plastid function by inhibiting metabolic pathways. Glyphosate is a broad-spectrum, postemergence herbicide that inhibits 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), a nuclear-encoded chloroplast-localized enzyme (Della-Cioppa et al., 1986) in the shikimic acid pathway of plants. Phosphinothricin (PPT) inhibits the chloroplast-localized enzyme Gln synthetase, resulting in a rapid buildup of ammonia and subsequent cell death (Tachibana et al., 1986). In previous work, we demonstrated that overexpression of CP4 in plastids could lead to extraordinarily high levels of EPSPS (>10% [w/w] of total soluble protein in leaves) and very high levels of herbicide tolerance (Ye et al., 2001). However, using selection parameters that work routinely for nuclear transformation, we were unable to obtain plastid transformants using CP4 as a selectable marker (G-N Ye, unpublished data). Likewise, resistance to PPT from a plastid-expressed bar transgene has been reported and transplastomic plants had field-level tolerance to the herbicide. However, direct selection of plastid transformants on PPT medium was not possible and transplastomic plants in these experiments were generated by antibiotic resistance (Lutz et al., 2001).
In an effort to understand the lethality of herbicides to plastids, we investigated their effects on plastid ultrastructure using transmission electron microscopy (TEM). The results show that levels of herbicide that can be used to routinely select nuclear transformants cause dramatic effects to plastid outer and internal membrane structures, resulting in plastid death. To circumvent this plastid lethality, we developed a “transformation segregation” scheme that uses a first selection on nonlethal antibiotic (spectinomycin) medium followed by a second selection on medium containing the herbicide. A cotransformation approach using two transformation vectors on separate plasmids, targeting aadA and either CP4 or bar to the plastid genome, allowed efficient recovery of transplastomic lines on herbicide-containing medium. Importantly, a high percentage of the transplastomic lines independently segregated the herbicide resistance marker to different genomes, thus allowing the recovery of herbicide resistant lines without concomitant integration of the antibiotic resistance marker. Furthermore, these studies indicate a high degree of persistence of unselected transforming DNA, providing useful insights into plastid chromosome dynamics.
RESULTS
Effects of Herbicide on the Ultrastructure of Tobacco Chloroplasts
Although the primary mode of action for glyphosate and PPT herbicides is to inhibit plastid-localized metabolic pathways, these effects alone could not explain the inability to directly select plastid transformants using the herbicide resistance genes. Therefore, we searched for secondary effects of herbicides on the ultrastructure of plastids, using TEM. To this end, tobacco leaf discs were cultured in the presence of selective levels of herbicide, or the antibiotic spectinomycin, for a period of 1 week to mimic the early stages of selection for plastid transformants. Tissues were then harvested, fixed, and processed for TEM analysis.
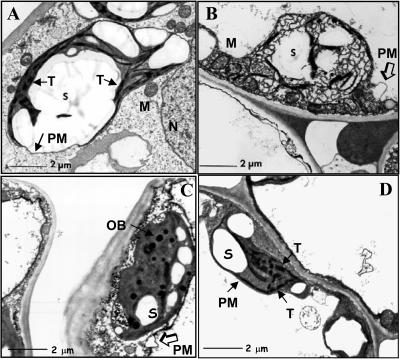
Figure 1 shows the ultrastructural features observed in representative cells of the cultured tobacco leaf discs. In cells from untreated controls, the typical morphology of metabolically active photosynthetic plastids can be seen (Fig. 1A). Highly reticulate internal membrane structure of thylakoids is seen including numerous regions of stacked grana. The bulk of the plastid volume is filled with starch. The stroma is smooth and of a normal density. The outer membrane of the plastid is clearly defined. The remainder of the cell shows numerous healthy mitochondria. In contrast to untreated controls, the glyphosate-treated cells (50 μm; Fig. 1B) have lost the reticulate network of thylakoid membranes, with some apparent membrane remnants scattered throughout the plastid stroma, indicating disintegration of the photosynthetic membranes. Furthermore, the outer plastid membrane has apparently ruptured in several locations (Fig. 1B), with plastid contents spilling out into the cell cytoplasm. Interestingly, the cytoplasm appears otherwise unaffected, and mitochondria that have obvious membrane structure are plentiful. The effects of PPT (4 mg L-1, Fig. 1C) on plastid ultrastructure are even more dramatic. Thylakoid membrane structures are completely absent. Instead, densely staining oil bodies are prevalent, indicating accumulation of toxic compounds. The outer plastid membrane was also ruptured in several places (Fig. 1C), with membranous structures leaking into the cytosol.
Figure 1.
Effect of glyphosate and PPT on tobacco chloroplasts by TEM. Leaves from in vitro-grown wild-type tobacco plants were cut into about 0.5-cm2 segments and were cultured for 1 week on Murashige and Skoog media or Murashige and Skoog media containing herbicide or spectinomycin antibiotic. The leaf pieces were then harvested, fixed, and prepared for TEM analysis. A representative micrograph from cultured leaf sections are shown. A, No selective agent. B, Fifty micromoles glyphosate. C, PPT (4 mg/L). D, Spectinomycin (500 mg/L). Note that the location of disrupted plastid outer membranes are indicated by open arrows. The scale is shown by the bar. N, Nucleus; S, starch granule; T, thylakoid; M, mitochondria; PM, plastid membrane; OB, oil body.
In contrast to the dramatic effects of the herbicides, the presence of spectinomycin antibiotic (500 mg L-1; Fig. 1D) had little effect on plastid ultrastructure. Thylakoid membranes were abundant and stacked into granal structures, and starch accumulation was similar to untreated controls. The outer plastid membrane appears unaffected. These results clearly show that the selective levels of herbicide used are lethal to plastids, whereas the selective level of antibiotic has no apparent detrimental effects during this early (1 week) phase of the selection process.
Plastid Transformation Segregation Scheme
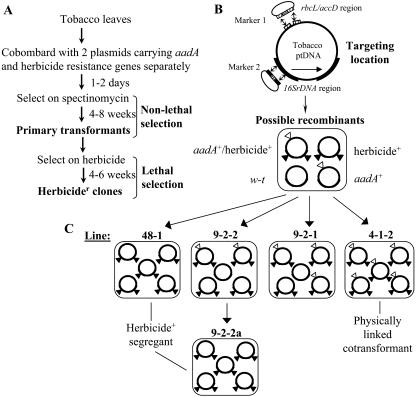
Figure 2 illustrates the “transformation segregation” scheme used to circumvent herbicide lethality and to recover independent transplastomic lines that carry exclusively the herbicide resistance marker and have segregated away the antibiotic resistance marker. The transformation segregation scheme consists of two phases of selection (Fig. 2A). The first, nonlethal phase of selection facilitates the recovery of plastid-transformed lines using the standard antibiotic (spectinomycin) selection protocol (Svab and Maliga, 1993). This phase of selection circumvents the exposure to lethal concentrations of herbicide during the initial critical stages of selection of plastid transformants. The spectinomycin resistant primary transformants recovered were then transferred to the second medium containing herbicide at a lethal concentration to recover those lines that had acquired the herbicide resistance gene. To achieve the subsequent segregation of the herbicide resistance gene away from the antibiotic resistance marker, the genes were delivered on two different transformation vectors, using a cobombardment approach. We anticipated that physical linkage of the cobombarded genes on the same transformed plastid genome would occur in some cases, as was shown previously (Carrer and Maliga, 1995). However, we also reasoned that a percentage of cotransformed lines might carry the transforming DNA integrated into independent plastid genomes that could subsequently segregate into independent plastid lineages. As shown in Figure 2B, the possible recombinants recovered by our transformation segregation scheme could include plastid-transformed lines carrying aadA and the herbicide resistance gene linked on the same genome (aadA+/herbicide+) or located on different genomes (aadA+, herbicide+). Furthermore, the initial herbicide-resistant clones could be heteroplasmic at the organelle and/or cellular level, with lines carrying a mixture of transformed genomes of either recombinant type along with wild-type genomes. Herbicide-resistant clones that were recovered through this process were further subjected to another round of plant regeneration on herbicide-containing medium to generate homoplasmic lines carrying only one type of plastid-transformed genome. Individual shoots that arose on herbicide medium from the same primary transformants are termed subclones. Multiple subclones for each independent line were analyzed for plastid genome content.
Figure 2.
Plastid transformation segregation scheme and diagram of possible plastid recombinants and herbicide resistant segregants. A, Plastid transformation segregation scheme for generation of herbicide+ segregants. Note that primary transformants are generated during the nonlethal selection phase and herbicide-resistant clones are identified during the subsequent lethal selection phase. B, Diagram of the targeting locations in the plastid genome (ptDNA) for the aadA and herbicide resistance markers (marker 1 and marker 2, respectively). Note that insertion of the transgenes is via homologous recombination through the flanking plastid DNA sequences. Possible recombinants are illustrated as residing in a single heteroplasmic organelle. Note that horizontal line indicates “copy correction” of transgene to both copies of the IR region (16SrDNA targeting region; heavy arcs). The rcbL/accD targeting region is in the LSC region of the plastid genome. W-t, Wild-type. C, Herbicide-resistant lines may be heteroplasmic as noted in the text. Lines that are aadA+/herbicide+ cotransformants or herbicide+ transformation segregants are recovered via ptDNA segregation and sorting after another round of plant regeneration. The plastid genome population in recovered plant lines as deduced by Southern probing is shown.
Recovery of Glyphosate-Resistant Transformation Segregants
Table I lists the selectable markers used for transformation and their targeting location in the plastid genome. We tested the efficiency of recovery of herbicide-resistant lines when the herbicide resistance marker was targeted to the LSC region or the IR region of the plastid genome, with the aadA gene targeted to the alternate location in each case.
Table I.
Cotransformation of tobacco plastids with aadA and herbicide resistance genes, and segregation of herbicide marker+ segregants.
Targets refers to the targeting location of the aadA and herbicide markers in the plastid genome. LSC, Large Single Copy region; IR: Inverted Repeat region of the tobacco plastid genome. # Specr and # Herb.r indicate the number of spectinomycin resistant or herbicide resistant events, respectively. % Cotransformation is expressed as the percentage of spectinomycin-resistant plastid transformants (excluding spontaneous mutants) that were also herbicide resistant. # Herb+ segregants is the number of transformation segregants containing only the herbicide resistance marker and no aadA gene after the lethal selection phase. % Segregants is expressed as the number of herbicide transformation segregants divided by the total number of herbicide-resistant transformants.
| Targetsa | #Specr | #Herb.r | %Co-transform. | #Herb+ segregants | % Segregants |
|---|---|---|---|---|---|
| aadA (LSC)/CP4 (IR) | 57 | 11 | 20 | 7 | 64 |
| aadA (IR)/CP4 (LSC) | 28 | 6 | 21 | 3 | 50 |
| aadA (LSC)/bar (IR) | 12 | 3 | 25 | 1 | 33 |
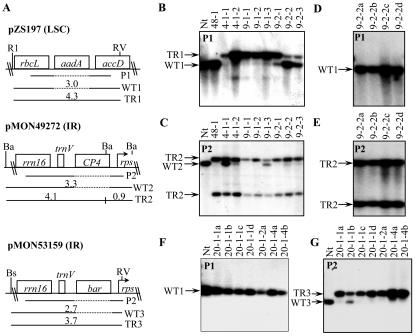
Transgenic lines arising after the herbicide selection phase were subjected to Southern-blot hybridization to confirm the presence or absence of the plastid transgenes in the subclones of the independent primary transformed lines. Table I summarizes the data from multiple independent experiments. Figure 3 shows examples of the result when the aadA gene was targeted to the LSC region (pZS197; Svab and Maliga, 1993) and the CP4 gene was targeted to the IR region (pMON49272) of the plastid genome (Fig. 3A). Insertion of the aadA gene into the LSC region produces a 4.3-kb DNA fragment (TR1) when digested with the restriction enzymes EcoRI and EcoRV (Fig. 3A), compared with a 3.0-kb DNA fragment (WT1) from wild-type genomes. Transgenic line 48-1 carried only wild-type (WT1) DNA in this region when probed for the aadA gene (Fig. 3B), indicating that the aadA gene has segregated away from the CP4 gene in this line. On the other hand, probing for the CP4 gene in the IR region produced two diagnostic bands of 4.1 and 0.9 kb (TR2; Fig. 3, A and C), with no detectable signal for the 3.3-kb wild-type band (WT2). Therefore, line 48-1 is homoplasmic for the CP4 gene and does not carry any aadA gene sequences, and is therefore a transformation segregant for the CP4 gene (Fig. 2C).
Figure 3.
Southern-blot analysis to confirm transformation segregation. A, Partial restriction maps of plastid DNA regions after transformation with vectors pZS197, pMON49272, or pMON53159. The chimeric aadA gene in pZS197 was targeted to the LSC region, whereas the chimeric CP4 and bar genes in vectors pMON49272 and pMON53159, respectively, were targeted to the IR region of the plastid genome. The probes (P) were derived from wild-type plastid DNA, and hybridize to wild-type (WT) or transplastomic (TR) DNA fragments of sizes indicated in the maps. Positions of resident plastid genes are shown. Total cellular DNA from multiple independent lines was digested with the appropriate restriction endonucleases as indicated, separated on a 0.8% (w/v) agarose gel, transferred to nitrocellulose, and probed. B through E, Transformation segregation approach using cotransformation of aadA targeted to the LSC region and CP4 targeted to the IR region of the plastid genome. Multiple independent cotransformed lines were analyzed for the presence of aadA-containing genomes (B and D; probe P1) or CP4-containing genomes (C and E; probe P2). F and G, Transformation segregation approach using cotransformation of aadA targeted to the LSC region and bar targeted to the IR region of the plastid genome. Subclones from the one line resistant to spectinomycin and PPT were analyzed for transgenome segregation after another round of growth on PPT-containing medium. Comparison of the different probings identifies the genome populations in each line and the presence of transformation segregants, as discussed in the text. Nt, Wild-type tobacco control.
In another example, the 9-2 line segregated subclones 9-2-1, 9-2-2, and 9-2-3 that were heteroplasmic for the integrated aadA gene. Subclone 9-2-1 carried less wild-type genomes (3.0-kb DNA fragment) than the aadA-containing genomes (4.3-kb DNA fragment), whereas subclones 9-2-2 and 9-2-3 carried more wild-type genomes (Fig. 3B). However, these subclones were nearly homoplasmic for the integrated CP4 gene (Fig. 3C). Therefore, at least three different populations of plastid genomes were present in these subclones: wild-type, aadA-containing genomes (with or without CP4) and CP4-only genomes (Fig. 2C). Additional rounds of regeneration on glyphosate-containing medium resulted in plants with a CP4-only genome (Fig. 3, D and E). Therefore, such lines represent additional CP4+ transformation segregant lines (Fig. 2C). Similar results were obtained (Table I) when targeting the aadA gene to the IR region and CP4 to the LSC region, indicating no apparent bias in genomic locations used for the transformation segregation scheme. In this case, six glyphosate-tolerant lines were produced after the second phase of selection, with three of these lines producing CP4+ transformation segregants.
Other lines and subclones, exemplified by lines 4-1-2, 9-1-1, 9-1-2, and 9-1-3, contained about equal proportions of aadA-containing and CP4-containing genomes as judged by hybridization intensities for these probes (Fig. 3, B and C), indicating that these are most likely cotransformed onto the same plastid genome (Fig. 2C). Physical linkage of unselected markers on the same plastid genome was predicted based on the previous observations (Carrer and Maliga, 1995).
Recovery of PPT-Resistant Transformation Segregants
To test the general applicability of the transformation segregation approach using another herbicide marker, we chose a chimeric bar gene that had previously been used unsuccessfully in direct selection experiments in tobacco plastids (Lutz et al., 2001). Likewise, our own attempts to use this construct for direct selection on PPT-containing medium failed (data not shown). The transformation segregation approach was applied using cobombardment of plasmids pMON53159 (Fig. 3A) carrying the chimeric bar gene and pZS197 carrying the aadA gene (Table I). From 60 bombarded plates, 12 spectinomycin-resistant transformed lines were obtained and subsequently transferred to PPT medium for selection of bar+ transformation segregants. Of the 12 transformants, one was shown to be resistant to 2 mg L-1 PPT. Multiple subclones for this line were generated during a subsequent round of plant regeneration on PPT-containing medium. Southern-blot analysis of these is shown in Figure 3, F through G. All of the subclones lost the aadA gene as evidenced by the wild-type pattern (WT1) after probing for the aadA gene. In contrast, probing for the bar region showed that all subclones carried the 3.7-kb TR3 hybridization pattern. Five out of the seven subclones tested (20-1-1c, 20-1-1d, 20-1-2a, 20-1-4a, and 20-1-4b) were homoplasmic for bar, indicating that they are bar+ transformation segregants.
Seed Germination Assay
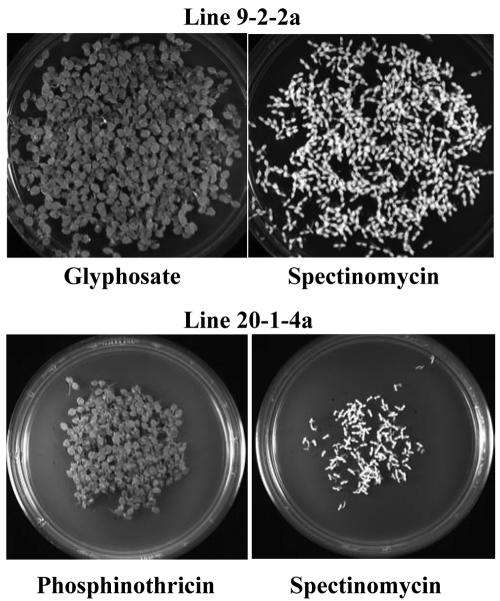
To ensure that the transformation segregants were free of aadA gene-containing plastid genomes, the antibiotic and herbicide resistance profiles of seedlings derived from multiple independent transformation segregant lines were analyzed. Subclones were grown to maturity in the greenhouse, seeds were harvested, and they were subsequently germinated on media containing 500 mg L-1 spectinomycin, 50 μm glyphosate, or 4 mg L-1 PPT. The seed resistance profile from several independent lines from glyphosate-resistant and PPT-resistant transformation segregants were assayed and indicated that 100% of selfed seed progeny were uniformly resistant to the herbicide treatment, as expected for homoplasmic plastid transformants (data not shown). As shown in the example in Figure 4, seedlings germinated uniformly green (resistant) on glyphosate- or PPT-containing medium, whereas seedlings from the same line germinated uniformly bleached (sensitive) on spectinomycin-containing medium. These results indicate that even though the original primary transformants were selected based on spectinomycin resistance, the transformation segregants were indeed free of the antibiotic resistance gene.
Figure 4.
Seedling germination assay. Seeds from homoplasmic selfed transplastomic lines 9-2-2a or line 20-1-4a selected in the glyphosate or PPT transformation segregation protocol, respectively, were surface-sterilized and germinated on medium containing 50 μm glyphosate, 4 mg/L PPT, or 500 mg/L spectinomycin. A, Note that all seedlings on the herbicide medium are green, indicating uniform resistance, whereas all seedlings are uniformly bleached on spectinomycin, indicating sensitivity. Photos were taken 10 d after plating seeds.
DISCUSSION
Experience with plant selectable markers indicates that selection can be lethal or nonlethal depending on the concentration of selective agent used, the timing of its application, and the relative expression level of the resistance transgene. The successful recovery of plastid transformants in tobacco depends on the ability to selectively amplify the one or very few plastid genome copies that are transformed in the initial bombardment event. Thus, previous work had suggested that nonlethal antibiotic (spectinomycin) selection is critical to this process (Maliga et al., 1993). Antibiotic selection using the aadA gene to confer spectinomycin resistance meets this criterion because cells respond only by bleaching and not by cell death (Maliga et al., 1993). In support of this, TEM analysis of leaf cell chloroplasts treated with antibiotic reveals no obvious deleterious effects (Fig. 1D). Therefore, we presume that aadA transgene expression levels are a less critical factor for success in the case of nonlethal spectinomycin selection. In contrast, treatment of leaf sections with the herbicides glyphosate and PPT at the concentration used here caused rapid and severe disintegration of inner and outer plastid membranes (Fig. 1), resulting in apparent plastid death. We conclude that the failure to directly select plastid transformants on these lethal levels of herbicide is due to loss of transformed plastids before they have a chance to accumulate sufficient transgene protein and therefore express phenotypic resistance to the herbicide. It may also be possible to overcome the lethal nature of herbicide treatment by modification of the selection conditions, as is illustrated by our more recent success in direct selection of glyphosate resistant events when the tissue is treated first at a low dose of glyphosate before transfer to the higher levels used here (G-N Ye and JM Staub, unpublished data).
Recent work further illustrates that the concentration of the selective agent and expression of the plastid transgene can be critical to the recovery of tobacco plastid transformants. Using a chimeric aphA-6 gene that confers resistance to kanamycin, Huang et al. (2002) obtained transformed shoots on 25 mg L-1 kanamycin-containing medium using a strong or a weak transgene promoter, along with numerous untransformed escape shoots. On the other hand, 50 mg L-1 of kanamycin was more selective and resulted in far fewer untransformed escape shoots, but transformants were obtained only if a strong promoter was used to drive the plastid transgene, and no transformants were obtained when the plastid transgene was driven by a weaker promoter. In contrast, earlier work by Carrer et al. (1993) used a chimeric nptII gene that allowed selection for plastid transformants on 50 mg L-1 kanamycin, but only at a very low efficiency and with numerous untransformed escape shoots produced. Although plastid ultrastructure was not examined in either of these reports, we speculate that the expression level of the aphA-6 gene in the recent work may have been high enough to circumvent any plastid-lethality caused by kanamycin, whereas expression levels of the nptII gene used in the earlier work may not have been as protective.
It should be noted that even the very well-expressed plastid CP4 and bar transgene constructs used in this work were not successful in direct selection experiments using the same protocols that work efficiently for nuclear transformation (this work; Lutz et al., 2001; Ye et al., 2001). We presume that this is due to the subcellular localization of the enzyme: in nuclear transformation, smaller amounts of the enzyme in the cytoplasm apparently provide for adequate plastid protection, whereas higher levels of transgene expression are required for protection when expressed from within the plastid compartment. This is consistent with our earlier finding that a nuclear-expressed CP4 transgene provides higher levels of cellular resistance to glyphosate at much lower protein concentration than does plastid-expressed CP4 even at very high levels of protein accumulation (Ye et al., 2001).
To circumvent the herbicide lethality seen here, a transformation segregation strategy was used that takes advantage of an initial nonlethal selection phase to recover plastid transformants, followed by a lethal selection to identify transplastomic lines that carry the transgene of interest (i.e. the herbicide resistance marker). A cobombardment approach to deliver the selective markers on different transformation vectors facilitated the segregation of transgenomes, and the subsequent recovery of homoplasmic lines carrying only the initially unselected herbicide marker. Plastid cotransformation using independently transforming markers has been reported previously (Kindle et al., 1991; Newman et al., 1991; Carrer and Maliga, 1995). However, in these cases, the unselected marker was most often recovered as physically linked to the transgenome carrying the selected marker and no attempt was made to selectively recover transformants that segregated the unselected transgene. In another example, cotransformation and subsequent segregation of the desired transgenome was performed by targeting of the selectable marker into an essential gene under selective conditions. The selectable marker-containing genomes could then be subsequently eliminated by removal of the selection pressure (Fischer et al., 1996). Linkage of cotransformed transgenes is valuable for increasing the effective insert size and number of integrated transgenes in plastid transformed lines. However, in addition, the transformation segregation approach allows separation of the desired transgene from the undesired antibiotic selectable marker. This application is clearly important for the generation of potentially commercial herbicide-resistant transplastomic plants that was not possible using a conventional direct selection approach (Lutz et al., 2001; G-N Ye and JM Staub, unpublished data).
Our observed cotransformation frequency of approximately 20% was predicted based on previous results in tobacco (Carrer and Maliga, 1995). However, we were surprised to recover such a high percentage of herbicide-resistant transformation segregation lines (in 50%-64% of cotransformed lines). Recovery of transformation segregants indicates that the unselected DNA must persist for a long period (approximately 4-8 weeks) until the second (lethal) selection phase is applied. Furthermore, the unselected genomes must already be amplified sufficiently at that time to prevent the rapid plastid lethality expected upon transfer of tissue to the lethal concentration of herbicide used in that phase.
Persistence of the unselected genomes may have occurred due to integration into different plastid genomes followed by coreplication with the selected genome population in plastid nucleiods (Nemoto et al., 1988; Sato et al., 1993), or as part of multimeric plastid genomes that could undergo subsequent resolution to the monomer form (Kolodner and Tewari, 1975; Deng et al., 1989; Bendich and Smith, 1990; Lilly et al., 2001). It is also very possible that intermolecular homologous recombination between cotransformed (physically linked) genomes and wild-type genomes in the same plastid occurred, resulting in potential segregant genomes. In an extreme case, for example, lines 9-2-1, 9-2-2, and 9-2-3 (Figs. 2C and 3), primary transformed lines were complex and carried a mixture of wild-type genomes, genomes cotransformed via physical linkage with both markers, and a mixture of potential segregant genomes. In other cases, for example, line 48-1 (Figs. 2C and 3), homoplasmic transformation segregants were obtained in the first subclone after transfer to herbicide medium. Regardless of the mechanisms that allowed persistence of the unselected genomes, plastid sorting during plant regeneration on herbicide-containing medium was ultimately responsible for generating the transformation segregants. Our results suggest that the transformation segregation strategy can be applied to any lethal selectable marker where direct selection is very difficult or impossible.
MATERIALS AND METHODS
TEM Analyses of Leaf Tissues Treated with Glyphosate or PPT
Tobacco (Nicotiana tabacum cv Petit Havana) leaf sections of about 0.5 cm2 were prepared from in vitro aseptically maintained plants and were placed on phytagel-solidified medium containing Murashige and Skoog salts (Murashige and Skoog, 1962), Gamborg's B5 vitamins (Gamborg et al., 1968), 3% (w/v) Suc, and various concentrations of glyphosate or PPT. To minimize plant to plant or leaf to leaf variations, leaf sections were placed randomly on media containing 50 μm glyphosate, 500 mg L-1 spectinomycin, or 2 or 4 mg L-1 PPT. Leaf sections were cultured at 24°C with a 16-h photoperiod for 1 week, and were then harvested for the analyses. As a control, wild-type leaf sections were grown on the same basal medium but without selective agent.
For TEM, cultured leaf tissues were cut into 1-mm2 pieces and fixed in 4% (w/v) glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.2, for 3 h, with the first 30 min under vacuum. The tissue was further fixed in 1% (w/v) osmium tetroxide in the above buffer for 90 min and was stained with 1% (w/v) aqueous uranyl acetate for 2 h. Ethanol and propylene oxide were used to dehydrate the tissue before infiltration with a 1:1 mixture of Spurr's: EMbed 812 resin. The resin was polymerized at 60°C for 48 h. The resulting blocks were sectioned on a microtome (Ultracut E; Leica, Deerfield, IL). Sections 80 nm thick were picked up on formvar/carbon-coated copper slot grids. The grids were stained with uranyl acetate and lead citrate in an Ultrastainer (LKB Instruments, Gaithersburg, MD) and were examined with a transmission electron microscope (1200; JEOL, Tokyo). All reagents were obtained from Electron Microscopy Sciences (Fort Washington, PA).
Construction of Plastid Transformation Vectors
The transformation vectors pZS197, pPRV112, and the bar cassette in pMON53159 were obtained from Dr. Pal Maliga (Rutgers University, Piscataway, NJ) and have been described (Svab and Maliga, 1993; Zoubenko et al., 1994; Lutz et al., 2001, respectively). Two vectors were constructed that carry the synthetic CP4 EPSPS chimeric gene derived from vector pMON49218 (Ye et al., 2001). The CP4 chimeric gene in pMON49272 was cloned between the trnV and rps12/rps7-3′ sequences used for homologous flanks for integration into the IR region of the tobacco plastid genome, or cloned between the rbcL and accD sequences as homologous flanks for integration into the LSC region.
Transformation and Phased Selection
Tobacco cv Petit Havana was maintained aseptically through cutting on phytagel-solidified medium containing Murashige and Skoog salts (Murashige and Skoog, 1962) and Gamborg's B5 vitamins (Gamborg et al., 1968) with 3% (w/v) Suc at 24°C with a 16-h photoperiod. Healthy and fully expanded leaves were harvested and bombarded with 0.6-μm gold particles coated with a mixture of transforming plasmids at a 1:1 ratio using a biolistic particle delivery system (PDS-1000/He; Bio-Rad, Hercules, CA) as described (Svab and Maliga, 1993; Ye et al., 2001). The bombarded leaves were cut into about 0.5-cm2 sections 1 d after bombardment and were selected on medium containing spectinomycin at 500 mg L-1 as described (Svab and Maliga, 1993; Staub and Maliga, 1994). Leaf sections from each spectinomycin-resistant line were placed onto medium with 50 μm glyphosate for glyphosate tolerance or PPT at 2 or 4 mg L-1. Leaf pieces were also screened on medium containing 500 mg L-1 each of spectinomycin and streptomycin to identify spontaneous mutants. Spectinomycin-resistant transformants were termed primary transformants. Those lines that gave rise to regenerated plants on glyphosate or PPT medium were considered as cotransformed. Individual shoots that regenerated on herbicide medium from the same primary transformant are termed subclones.
Southern-Blot Analysis
Isolation of total cellular DNA and Southern-blot hybridization were performed as described in Sidorov et al. (1999). Hybridization probes correspond to the tobacco plastid DNA fragments labeled in Figure 3; P1 is a PstI/XhoI DNA fragment and P2 is an EcoRV/EcoR1 DNA fragment.
Seed Germination Assay
Transformation segregant lines were grown in the greenhouse until maturity and seed pods were harvested. Seed assays for resistance to spectinomycin or glyphosate were performed as described (Svab and Maliga, 1993; Ye et al., 2001, respectively). Homoplasmic seedlings that are resistant to the selective agent are uniformly green on these media, whereas sensitive seedlings are bleached.
Acknowledgments
We thank Dr. Pal Maliga for plasmid clones, Drs. Ken Barton and Steve Padgette for support of this work, and Dr. Chuck Armstrong for critical review of the manuscript.
References
- Barry G, Kishore G, Padgette S, Taylor M, Kolacz K, Weldon M, Re D, Eichholtz D, Fincher K, Hallas L (1992) Inhibitors of amino acid biosynthesis: strategies for imparting glyphosate tolerance to crop plants. In BK Singh, HE Flores, and JC Shannon, eds, Biosynthesis and Molecular Regulation of Amino Acids in Plants. American Society of Plant Physiologists, Rockville, MD, pp 139-145
- Bendich AJ, Smith SB (1990) Structure of chloroplast and mitochondrial DNAs. Curr Genet 17: 421-425 [Google Scholar]
- Cao J, Dun X, McElroy D (1992) Regeneration of herbicide resistant transgenic rice plants following microprojectile-mediated transformation of suspension culture cells. Plant Cell Rep 11: 586-591 [DOI] [PubMed] [Google Scholar]
- Carrer H, Hockenberry TN, Svab V, Maliga P (1993) Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet 241: 49-56 [DOI] [PubMed] [Google Scholar]
- Carrer H, Maliga P (1995) Targeted insertion of foreign genes into the tobacco plastid genome without physical linkage to the selectable marker gene. Bio/Technology 13: 791-794 [Google Scholar]
- Clemente TE, Vallee BJ, Howe AR, Conner-Ward D, Rozman RJ, Hunter PE, Broyles DL, Kasten DS, Hinchee MA (2000) Progeny analysis of glyphosate selected transgenic soybeans derived from Agrobacterium-mediated transformation. Crop Sci Suppl 3 40: 797-803 [Google Scholar]
- Corneille S, Lutz K, Svab Z, Maliga P (2001) Efficient elimination of selectable marker genes from the plastid genome by the Cre-lox site-specific recombination system. Plant J Suppl 2 27: 171-178 [DOI] [PubMed] [Google Scholar]
- Daniell H, Muthukumar B, Lee SB (2001) Marker-free transgenic plants: engineering the chloroplast genome without the use of antibiotic selection. Curr Genet 39: 109-116 [DOI] [PubMed] [Google Scholar]
- Della-Cioppa G, Bauer SC, Klein BK, Shah DM, Fraley RT, Kishore G (1986) Translocation of the precursor of 5-enolpyruvylshikimate-3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci USA 83: 6873-6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XW, Wing RA, Gruissem W (1989) The chloroplast genome exists in multimeric forms. Proc Natl Acad Sci USA 86: 4156-4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Stampacchia O, Redding K, Rochaix J-D (1996) Selectable marker recycling in the chloroplast. Mol Gen Genet 251: 373-380 [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 50: 151-158, 1968 [DOI] [PubMed] [Google Scholar]
- Golds T, Maliga P, Koop H-U (1993) Stable plastid transformation in PEG-treated protoplasts of Nicotiana tabacum. Bio/Technology 11: 95-97 [Google Scholar]
- Hajdukiewicz PT, Gilbertson L, Staub JM (2001) Multiple pathways for Cre/lox-mediated recombination in plastids. Plant J Suppl 2 27: 161-170 [DOI] [PubMed] [Google Scholar]
- Huang F-C, Klaus SMJ, Herz S, Zou Z, Koop H-U, Golds TJ (2002) Efficient plastid transformation in tobacco using the aphA-6 gene and kanamycin selection. Mol Genet Genomics 268: 19-27 [DOI] [PubMed] [Google Scholar]
- Iamthan S, Day A (2000) Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat Biotechnol 18: 1172-1176 [DOI] [PubMed] [Google Scholar]
- Khan MS, Maliga P (1999) Fluorescent antibiotic resistant marker for tracking plastid transformation in higher plants. Nat Biotechnol 17: 910-915 [DOI] [PubMed] [Google Scholar]
- Kindle KL, Richards KL, Stern DB (1991) Engineering the chloroplast genome: techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 88: 1721-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R, Tewari KK (1975) Chloroplast DNA from higher plants replicates by both the Cairns and rolling circle mechanism. Nature 236: 708-711 [DOI] [PubMed] [Google Scholar]
- Lilly JW, Havey MJ, Jackson SA, Jiang J (2001) Cytogenomic analyses reveal the structural plasticity of the chloroplast genome in higher plants. Plant Cell 13: 245-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz KA, Knapp JE, Maliga P (2001) Expression of bar in the plastid genome confers herbicide resistance. Plant Physiol 25: 1585-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P, Carrer H, Kanevski I, Staub J, Svab Z (1993) lastid engineering in land plants: a conservative genome is open to change. Phil Trans R Soc Lond B 342: 203-208 [DOI] [PubMed] [Google Scholar]
- Mannerlof M, Tuvesson S, Steen P, Tenning P (1997) Transgenic sugar beet tolerant to glyphosate. Euphytica 94: 83-91 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for the growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473-497 [Google Scholar]
- Nemoto Y, Kawano S, Nakamura S, Mita T, Nagata T, Kuroiwa T (1988) Studies on plastid-nuclei (nucleoids) in Nicotiana tabacum L.: isolation of proplastid-nuclei from cultured cells and identification of proplastid-nuclear proteins Plant Cell Physiol Suppl 1 29: 167-177 [Google Scholar]
- Newman SM, Gillham NW, Harris EH, Johnson AM, Boynton JE (1991) Targeted disruption of chloroplast genes in Chlamydomonas reinhardtii. Mol Gen Genet 230: 65-74 [DOI] [PubMed] [Google Scholar]
- O'Neill C, Horvath GV, Horvath E, Dix PJ, Medgyesy P (1993) Chloroplast transformation in plants: polyethylene glycol (PEG) treatment of protoplasts is an alternative to biolistic delivery systems. Plant J Suppl 5 3: 729-738 [PubMed] [Google Scholar]
- Padgette SR, Re DB, Barry GF, Eichholtz DE, Delannay X, Fuchs RL, Kishore GM, Fraley RT (1996) New weed control opportunities: development of soybeans with a Roundup Ready gene. In SO Duke, Ed, Herbicide-Resistant Crops: Agricultural, Economic, Environmental, Regulatory and Technological Aspects. CRC Press, Boca Raton, FL, pp 53-84
- Ruf S, Hermann M, Berger I, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol 19: 870-875 [DOI] [PubMed] [Google Scholar]
- Sato N, Albrieux C, Joyard J, Douce R, Kuroiwa T (1993) Detection and characterization of a plastid envelope DNA-binding protein which may anchor plastid nucleoids. EMBO J Suppl 2 12: 555-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov VA, Kasten D, Pang S-Z, Hajdukiewicz PT, Staub JM, Nehra N (1999) Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J Suppl 2 19: 209-216 [DOI] [PubMed] [Google Scholar]
- Sikdar SR, Serino G, Chaudhuri S, Maliga P (1998) Plastid transformation in Arabidopsis thaliana. Plant Cell Rep 18: 20-24 [Google Scholar]
- Staub JM, Maliga P (1994) Translation of psbA mRNA is regulated by light via the 5′-untranslated region in tobacco plastids. Plant J 6: 547-553 [DOI] [PubMed] [Google Scholar]
- Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87: 8526-8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90: 913-917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Watanabe T, Sekizawa Y, Takematsu T (1986) Accumulation of ammonia in plants treated with biolaphos. J Pestic Sci 11: 33-37 [Google Scholar]
- Ye G-N, Hajdukiewicz PTJ, Broyles D, Rodriguez D, Xu CW, Nehra N, Staub JM (2001) Plastid-expressed 5-enolpyruvylshikimate-3-phosphate synthase genes provide high level glyphosate tolerance in tobacco. Plant J Suppl 3 25: 261-270 [DOI] [PubMed] [Google Scholar]
- Zhou H, Arrowsmith JW, Fromm ME, Hironaka CM, Taylor ML, Rodriguez D, Pajeau ME, Brown SM, Santino CG, Fry JE (1995) Glyphosate-tolerant CP4 and GOX genes as a selectable marker in wheat transformation. Plant Cell Rep 15: 159-163 [DOI] [PubMed] [Google Scholar]
- Zoubenko OV, Allison LA, Svab Z, Maliga P (1994) Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res 22: [DOI] [PMC free article] [PubMed]