Abstract
Antibodies were generated against the positively charged chair-like glycosidase inhibitor nojirimycin by in vitro immunization. A number of catalytic antibodies were isolated, one of which catalyzes the hydrolysis of p-nitrophenyl β-d-glucopyranoside 3 with a rate enhancement (kcat/kuncat) of 105 M over the HOAC-catalyzed reaction. The antibody discriminates modifications in the pyranoside ring of substrate 3 at the C2, C4, and the anomeric positions. The pH dependence of the reaction and chemical modification studies suggest the presence of an active-site Asp or Glu residue that may function as a general acid. This study further defines those requirements necessary to generate antibodies that efficiently cleave glycosidic bonds.
The development of biological catalysts that selectively cleave or form glycosidic bonds would contribute enormously to our ability to chemically manipulate the structures of carbohydrates (1). In addition, mechanistic and structural studies of these catalysts might provide new insights into the chemical requirements for this important transformation. One approach to this problem has been to exploit the structural diversity of the immune system to produce selective antibody (Ab) catalysts (2–6). This requires strategies for programming the combinatorial processes of the immune response with mechanistic information relating to the glycosidic bond cleavage reaction. Early efforts in this regard focused on cyclic acetals as substrates and antibodies that were generated to positively charged or conformationally restricted analogues of the oxocarbenium ion intermediate (7–9). The resulting antibodies accelerated the hydrolysis reaction (kcat/kuncat) from 100- to 1,000-fold.
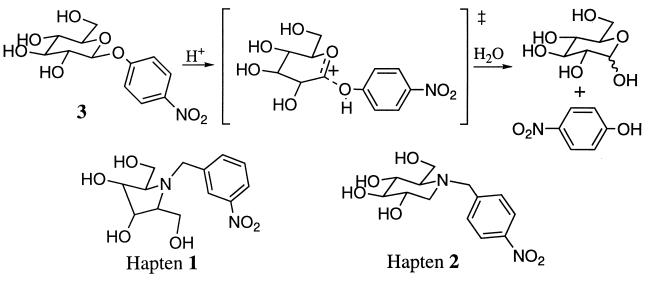
More recently, five-membered ring iminocyclitols (hapten 1) were used as transition-state analogues for the hydrolysis of aryl glucopyranosides and galactopyranosides (Fig. 1) (10, 11). The pyranose ring is thought to have a half-chair conformation in the transition state for glycosidic bond cleavage (12–18). Another distinctive feature of the transition state is the development of partial positive charge at the anomeric position. These structural and electronic features are both mimicked to some degree by the pyrrolidine ring of compound 1 (19). Antibodies specific for 1 catalyzed the hydrolysis of aryl pyranosides by a factor of roughly 104 over the uncatalyzed reaction (10, 11). To further investigate those structural features in a hapten that result in efficient antibody glycosidases, we have generated antibodies to a derivative of the natural glycosidase inhibitor nojirimycin (Fig. 1) (20, 21). In vitro immunization has resulted in the Ab 4f4f that catalyzes the hydrolysis of p-nitrophenyl β-d-glucoside with a rate enhancement (kcat/kHOAc) of 1.4 × 105 M.
Figure 1.
Glycosidic bond hydrolysis and the structures of haptens 1 and 2.
MATERIALS AND METHODS
Synthesis of Hapten 2.
To a stirred solution of deoxynojirimycin hydrochloride (30 mg, 0.15 mmol; Sigma) and K2CO3 (21 mg, 0.15 mmol) in dry dimethylformamide was added a solution of p-nitrobenzyl bromide (32 mg, 0.15 mmol) under an argon atmosphere at room temperature. The resulting solution was stirred an additional 18 h, and the solvent was removed. The residue was chromatographed on silica gel (methanol/ethyl acetate, 4:1; Rf = 0.4), affording 17 mg of hapten 2 as a white solid (yield 38%) (1):H NMR (300 MHz, CD3OD) δ 8.2 (d, 2H, J = 8 Hz), 7.6 (d, 2H, J = 8 Hz), 4.9 (s, 4H), 4.4 (m, 1H), 4.1 (m, 1H), 3.9 (m, 1H), 3.6 (m, 2H), 3.3 (m, 3H), 2.7 (m, 1H), 2.2 (m, 1H); 13C NMR (75 MHz, dimethyl sulfoxide-d6) δ 148.6, 146.7, 130.2 124.3, 79.2, 70.8, 68.9, 67.7, 56.8, 52.9, 47.8; HRMS (M+H)+ calcd., 299.1243; found, 299.1240.
In Vitro Immunization and Hybridoma Production.
Mouse spleen cells were isolated from an unprimed 10-week-old female BALB/c mouse and cultured in HY medium (DMEM supplemented with oxaloacetate, pyruvate, and insulin; Sigma) containing 10% fetal bovine serum (GIBCO/BRL) and supplemented with 20 μg (67 μM) of unconjugated hapten 2 for 3 days at 37°C in an atmosphere of 5% CO2/95% air (22). The cells were then fused with 107 P3X 63 Ag8.653 cells (American Type Culture Collection, CRL 1580) by using PEG (Sigma; molecular weight cut-off, 1,500) (23). The fused cells were selected in HY/10% fetal bovine serum medium supplemented with hypoxanthine/aminopterine/thymidine (Sigma) for 15 days and subsequently grown in medium supplemented with hypoxanthine/thymidine (Sigma). Supernatants from the cloned hybridoma cell lines were then screened for binding to hapten 2 by antigen-capture ELISA as described by Hornbeck (24). Unconjugated hapten 2 (10 μM) in 10 mM sodium phosphate/150 mM NaCl, pH 7.2 (PBS), was incubated in a 96-well ELISA plate at room temperature for 2 h, followed by blocking with 0.05% Tween 20. The supernatant from each hybridoma cell line was then added and the solutions were incubated overnight. The wells were then washed and incubated for 2 h with alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma), followed by the addition of p-nitrophenyl phosphate (Sigma). Absorbance (405 nm) of individual wells was measured by using a Molecular Device UV Max ELISA reader. Approximately 107 cells were injected into pristine-primed 12-week-old BALB/c mice to generate ascites fluid.
Ab Purification.
Ascites fluid (5–10 ml) was diluted with the same amount of binding buffer (1.5 M glycine/3.0 M NaCl, pH 8.9), filtered, and centrifuged to remove impurities. The crude Ab solution was applied to a protein G-agarose affinity chromatography column (1 cm × 4 cm). After washing with 10 vol of binding buffer, the Ab was eluted (2-ml fractions) with elution buffer (0.1 M glycine, pH 2.5), and the resulting eluant was neutralized with collection buffer (1.0 M Tris, pH 10.0). The eluted Ab was dialyzed into 50 mM Mes (pH 5.5) and further purified by ion-exchange chromatography using the cation-exchange resin SP-Sepharose (HiLoad 16/10; Pharmacia) as the stationary phase. For the mobile phase, buffer A (50 mM Mes, pH 5.5) and buffer B (50 mM Mes/1.0 M NaCl, pH 5.5) were used. Ab usually eluted at 30–35% buffer B and the purified Ab was then dialyzed into storage buffer (1.0 M sodium phosphate/150 mM NaCl/ 0.02% NaN3, pH 7.0).
Catalytic and Assays.
Initial screening for catalysis was carried out as follows: to 90 μl of Ab solution (2–3 mg/ml) in reaction buffer (10 mM Mes/100 mM NaCl, pH 4.5) was added 10 μl of 20 mM substrate stock solution to afford a final concentration of 2.0 mM substrate. The following six substrates were used: p-nitrophenyl α- and β-d-glucopyranoside, p-nitrophenyl α- and β-d-galactopyranoside, and p-nitrophenyl α- and β-mannopyranoside (Sigma). The assay mixture was incubated at 37°C and product conversion was measured by injecting 15-μl aliquots of the reaction mixture onto an HPLC and monitoring at 315 nm. A C18 column (Microsorb; 4.6 mm × 15 cm, 5 μm) was used as the stationary phase and 45% aqueous acetonitrile was used as the mobile phase. The amount of p-nitrophenol (4.0-min retention time at a flow rate of 0.8 ml/min) was determined by integration. Kinetic data was collected under conditions where less than 1% of the substrate was converted to the product by using low concentrations of Ab (0.1–0.2 mg/ml). To determine values of Ki (25), Ab solutions (80 μl) were mixed with hapten 2 dissolved in dimethyl sulfoxide (10 μl) and incubated for 5 min at room temperature. To the resulting solution, a stock solution of substrate 3 (10 μl) was added and the resulting assay mixture was incubated at 37°C. The final Ab concentration was 0.5 μM and the final substrate concentrations were 50 μM and 25 μM in 10 mM Mes/100 mM NaCl/10% dimethyl sulfoxide, pH 4.5. Background hydrolysis rates were measured under the same conditions used to assay Ab catalytic activity. Buffer salts included HCl/acetate (10 mM), acetate (10 mM), Mes (10 mM), EPPS (N-[2-hydroxyethyl]piperazine-N′-[3-propanesulfonic acid]; 10 mM), and Bicine (N,N-bis[2-hydroxyethyl]glycine; 10 mM) for pH values of 2–3, 3–4, 4–7, 7–8, and 8–9, respectively. The ionic strength was adjusted to 100 mM NaCl.
Chemical Modification.
To a stirred solution of Ab (0.1 mg/ml), either in the presence or absence of hapten 2 (125 μM), was added 200 vol of 0.4 M diazoacetamide solution (150 mM NaClO4/20 mM boric acid, pH 5.0) (26). The reaction was stirred for 8 h at room temperature, dialyzed into assay buffer (10 mM Mes/100 mM NaCl, pH 5.5), and concentrated for catalytic assays as described above.
RESULTS AND DISCUSSION
Design Considerations.
Glycosidic bond hydrolysis is catalyzed by several classes of relatively selective glycosidases. One of the most thoroughly characterized enzymes is hen egg-white lysozyme, which selectively hydrolyzes the linkage between C1 of N-acetylmuramic acid and C4 of N-acetylglucosamine (27). Structural, mutagenesis, and kinetic isotope effect studies (12–18, 27) suggest that the transition state has a large degree of SN1 character, with almost complete cleavage of the glycosidic linkage. As a result, partial rehybridization at C1 occurs, distorting the substrate into a half-chair conformation that resembles the high-energy oxocarbenium ion intermediate. The active-site residue Glu-35 in lysozyme has been proposed to facilitate the bond-breaking process by donating a proton to the glycosidic oxygen atom. In addition, van der Waals and electrostatic interactions with Asp-52 and other residues help stabilize the developing positive charge at C1 as well as the distorted conformation of the transition state. In contrast, the mechanisms of β-glucosidases are thought to be SN2-like based on analogous studies (28, 29) and direct detection of an α-glucosyl-enzyme intermediate (21).
Many reversible inhibitors have been designed to mimic the conformation or electrostatic character of the transition states of these glycosidases. In particular, δ-gluconolactone, an inhibitor of lysozyme (Ki = 200 μM), and δ-gluconolactam, an inhibitor of sweet almond β-glucosidase B (Ki = 29 μM), resemble the half-chair conformation but lack a positive charge (30, 31). The cyclic amine inhibitors including 1-deoxynojirimycin (Ki = 18 μM for sweet almond β-glucosidase), castanospermine, and australine rely on charge complementarity between the protonated nitrogen and negatively charged active-site carboxylates (20, 21). However, evidence suggesting that the nonprotonated species may be the true inhibitory agents casts some uncertainty on the precise mechanism of inhibition (30). The cyclic amidines (32), which combine the half-chair conformation and positive charge, inhibit β-glucosidase with a Ki of roughly 10 μM. The short lifetimes of the latter at physiological pH preclude their use as haptens to elicit catalytic antibodies (ref. 33 and J.Y., L. Hsieh and P.G.S., unpublished results).
To determine the degree to which charge complementarity alone can lead to antibodies with glycosidase activity, antibodies were generated to the simple N-benzylated 1-deoxynojirimycin derivative 2 (Fig. 1). The ammonium ion might be expected to induce a negatively charged glutamate or aspartate side chain in the active site (34) that can either act as a general acid or electrostatically stabilize the incipient oxocarbenium ion like transition state. The chair-like ring of compound 2 does not mimick a distorted half-chair transition state but does resemble the geometry of the pyranose substrates. The N-benzyl substituent was chosen for synthetic ease as well as the fact that models indicate the aryl substituent can adapt the same relative position as that in β-d-phenyl glucopyranoside.
Ab Generation and Screening.
Hapten 2 was synthesized by the reaction of 1-deoxynojirimycin hydrochloride with 4-nitrobenzyl bromide in the presence of mild base. The hapten was then used to generate mAbs by using in vitro immunization techniques that eliminate the need to conjugate the hapten to a carrier protein (35, 36). In the past we have attempted to generate catalytic antibodies by standard immunization methods with BALB/c and Swiss Webster mice using the same hapten conjugated to a number of different carrier proteins through the reduced p-nitro substituent. This process repeatedly led to small numbers of hybridomas (less than five) that produced hapten-specific antibodies, none of which were catalytic. This same result was observed in the case of the iminocyclitol hapten 1. However, when hapten 1 was immunized in vitro, a significant number of hapten-specific hybridomas were isolated, some of which had significant catalytic activity (11). Similarly, in vitro immunization with hapten 2 afforded roughly 20 hybridoma cell lines that produced Ab that specifically bound hapten as determined by ELISA, with a significant fraction having catalytic activity. Whether the difference between in vitro and in vivo immunization is associated with hapten conjugation or possible differences between the cellular processes involved in these immune responses is unclear at present.
Antibodies were purified both by affinity chromatography using protein G and cation-exchange chromatography, a process that we have found to effectively remove contaminating glycosidase activity. Because 1-deoxynojirimycin inhibits a broad range of glycoside-processing enzymes including β-glucosidases, α-glucosidases, and sucrase, a wide variety of reactions might be catalyzed by 1-deoxynojirimycin-specific antibodies. We therefore initially screened the antibodies for activity with p-nitrophenyl α- and β-d-glucopyranosides, p-nitrophenyl α- and β-d-galactopyranosides and p-nitrophenyl α- and β-d-mannopyranosides. Catalytic activity was found in antibodies from 13 hybridoma cell lines, among which Ab 4f4f showed the highest level of activity and was, therefore, chosen for further study.
Catalytic Activity and pH Dependence of Ab 4f4f.
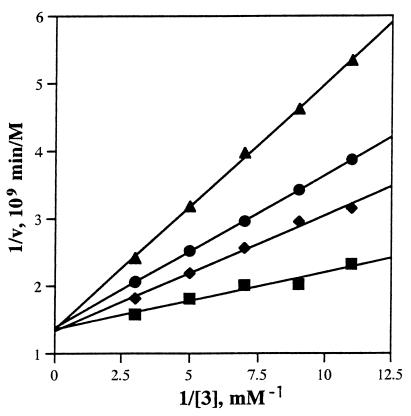
Ab 4f4f displayed saturation kinetics and the Michaelis constants were determined in 10 mM Mes/100 mM NaCl, pH 4.5. at 25°C (Fig. 2). The kcat and Km values for hydrolysis of p-nitrophenyl β-d-glucopyranoside are 2.8 × 10−3 min−1 and 22 μM, respectively, affording a value for kcat/Km of 130 M−1⋅min−1. The rate constants for the H+ (kH+), and OH− (kOH−) catalyzed reactions and the pseudo-first-order rate constant for the water (k′H2O) catalyzed reaction were also determined: k(H+) = 1.8 × 10−5 M−1⋅min−1, kOH− = 4.8 × 10−3 M−1⋅min−1, and k′H2O = 1.3 × 10−8 min−1. Thus the value of kcat/k′H2O for the Ab-catalyzed reaction is 2.2 × 105. For comparison the value of kAcOH for the acetic acid catalyzed reaction is 2 × 10−8 M−1⋅min−1. Thus Ab 4f4f is an efficient catalyst for the hydrolysis of substrate 3 and has a Vmax roughly 10-fold faster than either Ab generated against the iminocyclitol hapten 1 (10, 11); the kcat/Km value of Ab 4f4f is almost 100-fold higher than that of the hapten 1-specific Ab 24 (11). This result suggests that the positively charged ammonium ions in both haptens 1 and 2 play a key role in determining the catalytic activity of the resulting antibodies.
Figure 2.
Lineweaver–Burk plots of Ab 4f4f with none (▪), 39 nM (♦), 78 nM (•), and 160 nM (▴) hapten 2. The Ab solution was 0.77 μM.
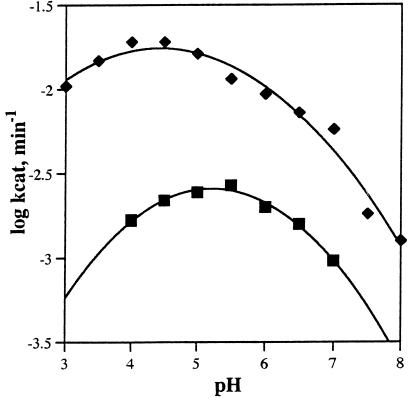
The pH dependence of the Ab-catalyzed reaction was examined by using two different substrates, p-nitrophenyl β-d-glucopyranoside 3 and p-nitrophenyl β-d-N-acetylglucosamide 8, between pH 4.0 and pH 8.0 (Fig. 3). Ab 4f4f has a maximum value of kcat at pH 5.3 for substrate 3 and pH 4.6 for substrate 8. These values and the decline in kcat at higher pH values are consistent with the presence of an active site group such as a Glu, Asp, or His that is active in its protonated form (34). Asp or Glu would likely be elicited in response to the positively charged ammonium ion of hapten 2. Indeed, it has been shown previously that a tertiary ammonium ion induces a negatively charged carboxylate in an Ab active site that acts as a general base in the elimination of HF from a β-fluoroketone (ref. 33; J.Y., L. Hsieh and P.G.S., unpublished results; and ref. 34). For Ab 4f4f, it is more likely that the carboxylate group is acting as a general acid. The decline in the value of kcat versus pH at low pH likely reflects the effects of pH on Ab structure and stability.
Figure 3.
The pH dependence of the catalytic activity (kcat) of Ab 4f4f. Two different substrates, p-nitrophenyl β-d-glucopyranoside (compound 3, ▪) and p-nitrophenyl N-acetyl-β-d-glucosaminide (compound 8, ♦) were used.
Substrate Specificity.
The kcat values of Ab 4f4f for the α- and β-aryl pyranoside substrates listed in Table 1 range from 5.2 × 10−4 min−1 to 2.8 × 10−3 min−1, corresponding to rate enhancements (kcat/k′H2O) of 1 × 105 to 2.5 × 105. On the other hand the values of Km varied from 22 μM for p-nitrophenyl β-d-glucopyranoside to 430 μM for p-nitrophenyl α-d-galactopyranoside. Thus it appears that the Ab stabilizes all of the transition states to a similar degree via either electrostatic or hydrogen bonding interactions (37) but varies significantly in its interactions with recognition elements in the substrates. In general, Ab 4f4f binds the β-anomers with higher affinity than the corresponding α-anomers, suggesting that the N-benzyl group of hapten 2 may better overlap the β-nitrophenol isomer of the pyranoside ring. Neither anomer of p-nitrophenyl d-mannopyranoside showed detectable hydrolysis, suggesting that equatorial substitution at the C2 hydroxyl group is required. At the same time other equatorial substituents at C2 are tolerated as indicated by the fact that p-nitrophenyl β-2-N-acetyl-d-glucosamide is a reasonably good substrate. The fact that d-galactopyranosides are good substrates indicates that the relative configuration of the C4 hydroxyl group is not critical to catalysis (but does influence binding of substrate). However, substitution of the C4 hydroxyl with additional monomers resulted in a complete loss of catalytic activity; i.e., there was no detectable hydrolysis of either anomer of p-nitrophenyl β-d-cellobioside by Ab 4f4f. Thus it is likely that the substrates and hapten 2 are bound in an active site cavity, as is the case in many small molecule–Ab complexes (39). However, there appears to be a relatively loose fit with many regions of the substrate perhaps due to the presence of active site water molecules or a relatively large degree of solvent accessibility.
Table 1.
Catalytic constants of Ab 4f4f and Ab 248 for various substrates
| Antibody | Substrate | kcat, min−1 | Km, μM | kcat/kuncat, × 10−5 | kcat/Km, M−1⋅min−1 |
|---|---|---|---|---|---|
| Ab 4f4f | p-Nitrophenyl β-d-glucopyranoside (3) | 2.8 × 10−3 | 22 | 2.0 | 130 |
| Ab 24 | p-Nitrophenyl β-d-glucopyranoside (3) | 3.2 × 10−4 | 160 | 0.25 | 1.7 |
| Ab 4f4f | p-Nitrophenyl α-d-glucopyranoside (4) | 6.2 × 10−4 | 170 | 2.5 | 3.7 |
| Ab 4f4f | p-Nitrophenyl β-d-galactopyranoside (5) | 3.0 × 10−3 | 190 | 1.1 | 16 |
| Ab 4f4f | p-Nitrophenyl α-d-galactopyranoside (6) | 1.2 × 10−3 | 430 | 2.0 | 2.8 |
| Ab 4f4f | p-Nitrophenyl β-d-fucopyranoside (7) | 5.3 × 10−4 | 80 | 1.0 | 6.7 |
| Ab 4f4f | p-Nitrophenyl β-d-N-acetylglucosamide (8) | 2.0 × 10−2 | 110 | 0.21 | 180 |
kuncat value is k′H2O.
Inhibition and Chemical Modification Studies.
The glycosidase activity of Ab 4f4f toward substrate 3 was competitively inhibited by the addition of hapten 2 to the reaction. The Ki value of 2 was determined to be 0.2 μM in 10 mM Mes/100 mM NaCl, pH 4.5, at 37°C. Thus, Ab 4f4f binds its hapten with considerably higher affinity than that of the most efficient catalytic antibodies (Ab 24, Ki (compound 1) = 380 μM; Ab 21, Ki (compound 1) = 60 μM) previously generated by in vitro immunization with iminocyclitol 1 (11). Clearly there is not a direct correlation of rate enhancement with preferential binding of hapten 2 relative to substrate 3. This may reflect the role of an active-site carboxylate group as a general acid rather than acting to simply electrostatically stabilize the transition state.
To determine whether an aspartate or glutamate residue plays a role in catalysis, chemical modification experiments were performed with the carboxylate specific reagent diazoacetamide (26). Ab 4f4f was treated with diazoacetamide in the presence and absence of 125 μM hapten 2, followed by extensive dialysis and catalytic assays with substrate 3. Modification in the presence of hapten 2 resulted in a 3% loss in activity, but in the absence of hapten, 92% of the catalytic was destroyed. These results again suggest the presence of an active site carboxylate and are consistent with the pH-dependent behavior of the Ab-catalyzed reaction. Additional experiments including kinetic isotope effects experiments, structural studies, and mutagenesis studies are required to further define the mechanism of the glycosidic bond cleavage reaction.
Acknowledgments
This work was financially supported by the Korea Institute of Science and Technology and the Korea Ministry of Health and Welfare. P.G.S. is a Howard Hughes Medical Institute Investigator.
ABBREVIATION
- Ab
antibody
References
- 1.Dwek R A, Edge C J, Harvey D J, Wormald M R, Parekh R B. Annu Rev Biochem. 1993;62:65–100. doi: 10.1146/annurev.bi.62.070193.000433. [DOI] [PubMed] [Google Scholar]
- 2.Schultz P G, Lerner R A. Science. 1995;269:1835–1842. doi: 10.1126/science.7569920. [DOI] [PubMed] [Google Scholar]
- 3.Schultz P G, Lerner R A. Acc Chem Res. 1993;26:391–395. [Google Scholar]
- 4.Jacobsen W H, Scanlan T S. Annu Rev Biophy Biomol Struct. 1997;26:461–493. doi: 10.1146/annurev.biophys.26.1.461. [DOI] [PubMed] [Google Scholar]
- 5.Tramontano A, Janda K D, Lerner R A. Science. 1986;234:1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 6.Pollack S J, Jacobs J W, Schultz P G. Science. 1996;234:1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- 7.Reymond J-L, Janda K D, Lerner R A. Angew Chem Int Ed Engl. 1991;30:1711–1713. [Google Scholar]
- 8.Suga H, Tanimoto N, Sinskey A J, Masamune S. J Am Chem Soc. 1994;116:11197–11198. [Google Scholar]
- 9.Yu J, Hsieh L, Kochersperger L, Yonkovich S, Stephans J C, Gallop M A, Schultz P G. Angew Chem Int Ed Engl. 1994;33:339–341. [Google Scholar]
- 10.Janda K D, Lo L-C, Shin M-M, Wang R, Wong C-H, Lerner R A. Science. 1997;275:945–948. doi: 10.1126/science.275.5302.945. [DOI] [PubMed] [Google Scholar]
- 11.Yu, J. & Schultz, P. G. (1997) J. Chem. Soc. Chem. Commu., in press.
- 12.Rosenberg S, Kirsch J F. Biochemistry. 1981;20:3196–3204. doi: 10.1021/bi00514a032. [DOI] [PubMed] [Google Scholar]
- 13.Smith L E H, Mohr L H, Raftery M A. J Am Chem Soc. 1973;95:7497–7500. doi: 10.1021/ja00803a046. [DOI] [PubMed] [Google Scholar]
- 14.Malcolm B A, Rosenberg S, Corey M J, Allen J S, de Baetselier A, Kirsch J F. Proc Natl Acad Sci USA. 1989;86:133–137. doi: 10.1073/pnas.86.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki R, Weaver L H, Matthews B W. Science. 1993;262:2030–2033. doi: 10.1126/science.8266098. [DOI] [PubMed] [Google Scholar]
- 16.Amyes T L, Jencks W P. J Am Chem Soc. 1989;111:7888–7900. [Google Scholar]
- 17.Withers S G, Street I P. J Am Chem Soc. 1988;110:8551–8553. [Google Scholar]
- 18.Schramm V L, Horenstein B A, Kline P C. J Biol Chem. 1994;269:18259–18262. [PubMed] [Google Scholar]
- 19.Liu K C. J Org Chem. 1991;56:6280. [Google Scholar]
- 20.Bause E, Legler G. Hoppe-Seyler’s Z Physiol Chem. 1974;355:438–442. doi: 10.1515/bchm2.1974.355.1.438. [DOI] [PubMed] [Google Scholar]
- 21.Withers S G, Street I P. J Am Chem Soc. 1988;110:8551–8553. [Google Scholar]
- 22.Stahl M, Goldie B, Bourne S P, Thomas N R. J Am Chem Soc. 1995;117:5164–5165. [Google Scholar]
- 23.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. p. 196. [Google Scholar]
- 24.Hornbeck P. Current Protocols in Immunology. New York: Wiley; 1992. pp. 2.1.1–2.1.22. [Google Scholar]
- 25.Dixon M. Biochem J. 1953;55:170–175. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossberg A L, Pressman D. J Am Chem Soc. 1960;82:5478–5482. [Google Scholar]
- 27.Sinnott M L. Chem Rev. 1990;90:1171–1202. [Google Scholar]
- 28.Hiatt A, Cafferkey R, Bowdish K. Nature (London) 1989;342:76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- 29.Dahlquist F W, Rand-Meir T, Raftery M A. Biochemistry. 1969;8:4214–4221. doi: 10.1021/bi00838a045. [DOI] [PubMed] [Google Scholar]
- 30.Dale M P, Ensley H E, Kern K, Sastry K A R, Byers L D. Biochemistry. 1985;24:3530–3539. doi: 10.1021/bi00335a022. [DOI] [PubMed] [Google Scholar]
- 31.Secemski I I, Leherer S S, Lienhard G E. J Biol Chem. 1972;247:4740–4748. [PubMed] [Google Scholar]
- 32.Tong M K, Papandreou G, Ganem B. J Am Chem Soc. 1990;112:6137–6139. [Google Scholar]
- 33.Shokat K M. Ph.D. thesis. Berkeley: Univ. of California; 1991. [Google Scholar]
- 34.Shokat K M, Leumann C J, Sugasawara R, Schultz P G. Nature (London) 1989;338:269–271. doi: 10.1038/338269a0. [DOI] [PubMed] [Google Scholar]
- 35.Borrebaeck C A. Trends Biotechnol. 1986;4:147. [Google Scholar]
- 36.Borrebaeck C A. J Pharm Biomed Anal. 1897;5:783. doi: 10.1016/0731-7085(87)80096-1. [DOI] [PubMed] [Google Scholar]
- 37.Warshel A. Proc Natl Acad Sci USA. 1978;75:5250–5254. doi: 10.1073/pnas.75.11.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson I A, Stanfield R L. Curr Opin Struct Biol. 1993;3:113–118. [Google Scholar]
- 39.Patten P A, Gray N S, Yang P L, Marks C B, Wedemayer G, Boniface J, Stevens R, Schultz P G. Science. 1996;271:1086–1091. doi: 10.1126/science.271.5252.1086. [DOI] [PubMed] [Google Scholar]
- 40.Wedemayer G J, Patten P A, Wang L H, Schultz P G, Stevens R C. Science. 1997;276:1665–1669. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]