Abstract
The bacterial cell division protein FtsZ assembles into the cytokinetic Z ring that directs cytokinesis in prokaryotes. In Escherichia coli the formation of the Z ring is prevented by induction of the cell division inhibitor SulA (SfiA), a component of the SOS response. Here we show that a MalE-SulA fusion that retains this inhibitory function in vivo inhibits the GTPase activity and polymerization of FtsZ in vitro. MalE-SulA10, which does not block Z ring formation in vivo, is unable to inhibit the GTPase activity and polymerization in vitro. Furthermore, FtsZ114, which is refractory to SulA in vivo, is not inhibited by MalE-SulA. These results indicate that SulA blocks Z ring formation by blocking FtsZ polymerization.
Keywords: cytokinesis, GTPase, tubulin, polymerization
Cytokinesis and chromosome segregation are temporally and spatially well coordinated in Escherichia coli, ensuring that very few DNA-less cells are formed (1). Although the mechanism of chromosome segregation in bacteria is largely unknown, the process of septation occurs through the action of the cytokinetic Z ring (2, 3), which forms at midcell about the time that chromosomes are visibly segregated (4, 5). The Z ring is a cytoskeletal element that recruits other proteins to the septum and directs invagination of the septum (6–9).
FtsZ has weak but significant homology to tubulin, suggesting that it is an ancestral homologue (10–13). In vitro FtsZ has GTPase activity (10, 13, 14) and polymerizes into protofilaments that further assemble into sheets or bundles of protofilaments, depending on the conditions (11, 15, 16). Polymerization is promoted by cationic agents such as DEAE-dextran and cationic lipids and occurs with GDP or GTP. We have, however, defined conditions in which FtsZ polymerization occurs efficiently in the absence of these agents and is strictly GTP-dependent (16). Importantly, FtsZ polymers formed under these latter conditions are, like microtubules, dynamic due to GTP hydrolysis. These in vitro dynamics of FtsZ polymers provide a basis for the in vivo dynamics of the Z ring (16).
When DNA is damaged the SOS response is induced and cell division is inhibited (reviewed in ref. 17). In part, this inhibition is due to the induction of sulA (sfiA), one of the SOS genes (18). SulA is expressed after DNA damage and, because it is very unstable, rapidly disappears once the DNA is repaired (19). Its rapid disappearance coincides with a rapid resumption of cell division, even in the absence of protein synthesis, indicating that SulA does not irreversibly damage the cell division machinery (20). The inhibition by SulA is prolonged in a lon mutant in which the degradation of SulA is reduced (19). As a result lon mutants are hypersensitive to DNA damage, as the stabilized SulA leads to prolonged filamentation and cell death (21). Suppressors of lon have been isolated, and they map either in the sulA gene or in the ftsZ gene (22–25). This genetic result led to the suggestion that ftsZ is the target of sulA (22). Consistent with this, overproduction of FtsZ also suppresses lon (26).
Induction of SulA, even in the absence of DNA damage, inhibits formation of the Z ring (27). Several approaches indicate that the mechanism for this inhibition involves a direct interaction between FtsZ and SulA. In one approach the yeast two-hybrid system was used to show that FtsZ and SulA interact directly (28). The significance of this interaction was supported by introducing various sulA or ftsZ mutations into this test system. Most of these mutations, which either inactivate sulA or make ftsZ refractory to the induction of sulA, eliminate the interaction observed between SulA and FtsZ. In another approach, Horiuchi and colleagues (29) fused SulA to MalE to facilitate purification. This fusion retained an inhibitory function in vivo and allowed for purification of the protein. It was observed that this fusion bound to FtsZ in a manner that required GTP hydrolysis. However, the fusion had no effect on the GTPase activity of FtsZ.
In addition to the wild-type FtsZ a number of mutant FtsZ proteins have been studied. The product of the temperature-sensitive allele, ftsZ84 (Ts), has been characterized and found to have reduced GTPase activity at all temperatures, but it displays ATPase activity (10, 13, 30). The other mutant proteins that have been characterized are products of alleles of ftsZ that display resistance to SulA (31). Most of these display dramatically reduced GTPase activity; however, they support cell growth. Only one, FtsZ114, displays significant GTPase activity in vitro, about 50% of the wild-type activity. If the GTPase activity is essential in vivo then it is not clear why most of these mutant proteins are inactive in vitro.
In this study we further explore the interaction between FtsZ and SulA by exploiting the MalE-SulA fusion. We find that the MalE-SulA fusion inhibits the GTPase activity of FtsZ and that this inhibition is abrogated by introduction of a mutant SulA into the fusion. Furthermore, we find that the GTPase activity of FtsZ114, which was isolated on the basis of resistance to SulA, is resistant to MalE-SulA. Importantly, we find that the MalE-SulA fusion blocks the polymerization of FtsZ. Thus, SulA is likely to inhibit Z ring formation in vivo by preventing the polymerization of FtsZ.
MATERIALS AND METHODS
Strains and Plasmids.
The E. coli strain DH5α (GIBCO/BRL) was used as a cloning host and to assess the effects of the malE-sulA fusions. The malE-sulA fusions were constructed in the expression vector pBAD18 (32). First, the malE gene was obtained from pMalC (New England Biolabs) by PCR and inserted into the NheI and HindIII sites of pBAD18 to give pJC90. The primers were designed such that the ribosome-binding site of malE and the restriction sites, including EcoRI at the 3′ end of malE, were retained. In a second step sulA alleles were obtained by PCR and inserted into the EcoRI and HindIII sites of pJC90 so that they would be in frame with the 3′ end of malE. pJC93 contained sulA, pJC94 contained sulA10, and pJC95 contained sulA54 fused to malE. The sources of the sulA alleles were the yeast two-hybrid plasmids constructed previously (28). The sulA alleles were sequenced to confirm that no mutations were introduced during PCR.
Bacterial Growth.
DH5α was grown in L broth with the addition of ampicillin (100 μg/ml) for the selection of plasmids. Glucose (0.4%) was added to depress the expression of Pbad, and arabinose was used at various concentrations to induce its expression. For experiments to determine the effect of MalE-SulA fusions on cell morphology and Z ring formation, cultures of DH5α containing the appropriate plasmid were grown overnight in L broth with ampicillin and glucose. The following day the strains were subcultured in the same media and grown for several hours and again subcultured. Arabinose was added at various concentrations and samples were taken at 1 hr for determination of the levels of the fusions and for immunofluorescence microscopy to see the effect on Z rings (4).
Immunoblotting and Immunofluorescence.
The levels of FtsZ and the MalE-SulA fusions were determined by immunoblotting. Cultures of DH5α containing pJC93 or pJC94 were grown in the presence of glucose as described above and induced for 1 hr with various concentrations of arabinose. Samples were taken at 1 hr and run on SDS/PAGE and transferred to nitrocellulose. FtsZ and MalE-SulA fusions were detected by using antibodies to FtsZ and MalE (New England Biolabs) with a secondary antibody conjugated to alkaline phosphatase (33). The levels were determined by comparison to known amounts of the same proteins run on the same gel.
Purification of the MalE-SulA Fusions.
The MalE-SulA fusions were purified from cultures of DH5α containing pJC93 or pJC94 after induction with arabinose for 3 hr. Although the overnight cultures were grown with glucose it was removed by centrifugation before induction with arabinose. After induction, cells were collected by centrifugation and lysed with a French press. Cell debris and membranes were removed by low-speed and high-speed centrifugations, respectively, and the fusions were purified from the supernatant by amylose column chromatography as described by the supplier (New England Biolabs) except that 20 mM Hepes–NaOH (pH 7.2) was used instead of phosphate buffer. Protein was estimated by Protein Assay reagent (Bio-Rad) using BSA as a standard.
Purification and Assays of FtsZ.
The FtsZ was purified as recently described (16). FtsZ114 was over-expressed from a derivative of the expression vector pJF118 containing ftsZ114 (31) and purified by the same procedure. The GTPase activity was determined by the release of labeled Pi from [γ-32P]GTP (34). The procedures for monitoring FtsZ polymerization by negative-stain electron microscopy and sedimentation have been described (16). Polymerization was carried out in polymerization buffer (50 mM Mes, pH 6.5/10 mM MgCl2, with either 50 or 200 mM KCl as indicated) at 30°C for samples examined by electron microscopy and 25°C for samples monitored by centrifugation. Polymerization was initiated by the addition of GTP and shifting to the incubation temperature. The amount of FtsZ present in polymers (pellets after centrifugation) was determined by running samples in SDS/PAGE along with known amounts of FtsZ. The Coomassie blue-stained FtsZ bands were quantitated by using a Personal Densitometer SI with ImageQuant software (Molecular Dynamics).
RESULTS
Characterization of MalE-SulA Fusions.
To study the interaction between FtsZ and SulA we used the approach of Higashitani et al. (29), who fused SulA to MalE. As controls we used fusions of MalE to mutant SulA proteins, SulA10 and SulA54, that do not inhibit division and do not interact with FtsZ when tested in the yeast two-hybrid system (28). We found that the malE-sulA fusion must be quite toxic, as we were unable to construct it in a multicopy plasmid downstream of the tac promoter (pJF118HE), although we were able to construct malE-sulA10 and malE-sulA54 fusions in the same vector. Therefore we constructed the malE-sulA fusion on a multicopy plasmid downstream of the arabinose promoter in pBAD18 to give pJC93. The arabinose promoter is more tightly regulated than the tac promoter (32).
In the presence of glucose the cell morphology of DH5α (pJC93) was fairly normal, however, in the absence glucose, even without the addition of arabinose, the basal level of expression of the fusion was inhibitory and cells were filamentous. The addition of arabinose to 0.001% led to more extensive filamentation and poor growth, and increasing the concentration to 0.005% inhibited colony formation (Table 1). In contrast, cells containing the plasmids pJC94 or pJC95, pBAD18 derivatives expressing MalE-SulA10 and MalE-SulA54, respectively, had normal morphology in the absence of arabinose and were able to form colonies at arabinose concentrations up to 0.05% (Table 1). At very high arabinose concentrations growth was inhibited. These results confirmed that the malE-sulA fusion retained the ability to inhibit division as reported previously (29). In addition, it demonstrated that the sulA10 and sulA54 mutations dramatically attenuate the inhibitory activity of the MalE-SulA fusion. Inhibition of cell growth by these fusions at high arabinose concentrations could be due to residual activity of the fusion to inhibit division or nonspecific interactions of the fusion expressed at such high levels. Even MalE inhibits division to some extent when expressed at high concentrations (unpublished observations).
Table 1.
Effect of MalE-SulA fusions on the growth of DH5α
| Arabinose | pJC93 (MalE-SulA) | pJC94 (MalE-SulA10) | pJC95 (MalE-SulA54) |
|---|---|---|---|
| None, glucose | ++ | ++ | ++ |
| None | ++* | ++ | ++ |
| 0.0005% | + | ++ | ++ |
| 0.001% | + | ++ | ++ |
| 0.005% | − | ++ | ++ |
| 0.05% | − | ++ | ++ |
| 0.5% | − | − | − |
DH5α containing these plasmids was streaked on L agar plates containing 100 μg/ml ampicillin and incubated overnight at 30°C. ++, Indicates normal growth; +, colony formation was poor and cells were extremely filamentous.
Colony formation was normal; however, the cells were a mixture of filamentous and normal-sized cells.
To further monitor the effect of the MalE-SulA fusions on cell morphology, DH5α containing either pJC93 or pJC94 was grown in the presence of glucose. At time 0 arabinose was added at various concentrations, and samples were taken 1 hr later to examine cell morphology and to determine the levels of FtsZ and the fusion proteins. Because glucose was present in the medium a higher concentration of arabinose was required to cause filamentation than was present during the viability measurements. With pJC93 filamentation was observed at 1 hr after the addition of 0.05% arabinose and Z rings were absent, indicating that MalE-SulA prevents Z ring formation (Fig. 1A). Interestingly, some of the cells from the culture contained invaginations without Z rings (indicated by the arrows), indicating that MalE-SulA also caused assembled Z rings to abort. At lower arabinose concentrations filamentation was not observed (data not shown). With pJC94 no filamentation was observed at 0.05% arabinose and Z rings were present (Fig. 1A).
Figure 1.
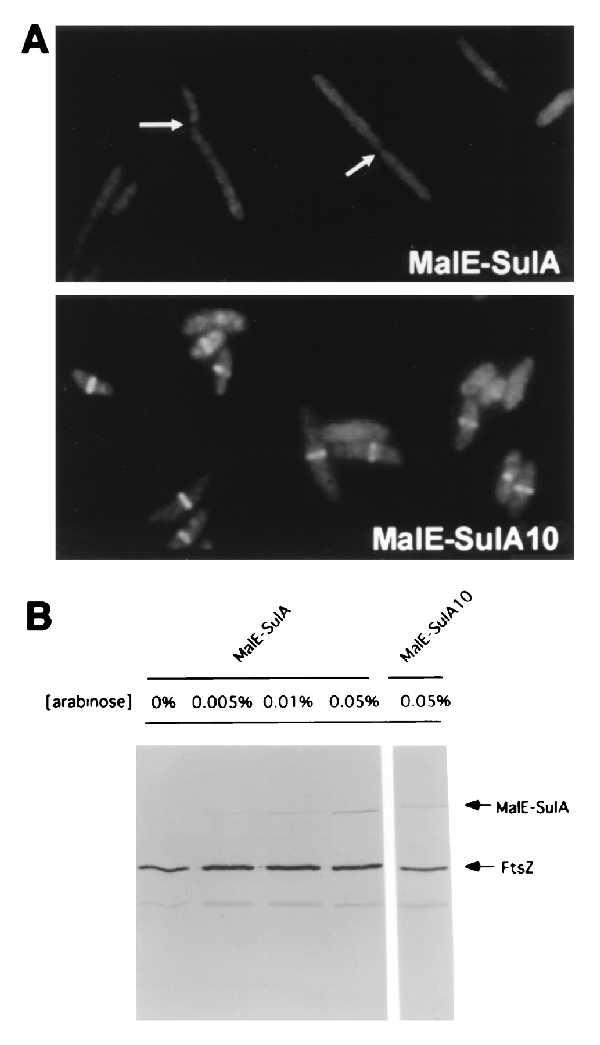
Cell morphology and Z ring formation after induction of MalE-SulA fusions. (A) Cultures of DH5α (pJC93) or (pJC94) were grown in L broth with glucose. Arabinose was added at 0.05% and incubation was continued for 1 hr. Samples were taken and cells were immunostained with antibodies to FtsZ. The arrows indicate cells that have constrictions but lack Z rings. (×1,000.) (B) The induction of the MalE-SulA fusions monitored by immunoblot analysis. Samples from the cultures in A as well as several additional cultures with less arabinose were taken for immunoblotting. The samples were separated by SDS/PAGE, transferred to nitrocellulose, and immunostained with antibodies against FtsZ and the MalE-SulA fusion. The bands corresponding to FtsZ and the MalE-SulA fusions are indicated.
Immunoblotting of lysates of cells taken 1 hr after arabinose addition allowed us to determine the level of MalE-SulA that was induced and to compare this with the level of FtsZ (Fig. 1B). With 0.01% arabinose little filamentation was observed at 1 hr and less MalE-SulA was present than with 0.05% arabinose. With 0.05% arabinose the molar ratio of MalE-SulA to FtsZ was 1:8. The MalE-SulA10 fusion was induced to the same level as the wild type.
Examination of the Effect of MalE-SulA Fusions on GTPase Activity of FtsZ.
The MalE-SulA fusions were purified by amylose affinity column chromatography and tested for their effect on the GTPase activity of FtsZ. Fig. 2A shows the effect of increasing the concentration of MalE-SulA on the GTPase activity of FtsZ. At a molar ratio of the fusion to FtsZ of 1.25:1 (300 μg/ml MalE-SulA) the GTPase activity is inhibited by 50%. As this molar ratio is increased to 2.5:1 the inhibition is increased to approximately 90%. This finding contrasts dramatically with the results of Higashitani et al. (29), who reported no inhibition of GTPase activity at a molar ratio of 2:1.
Figure 2.
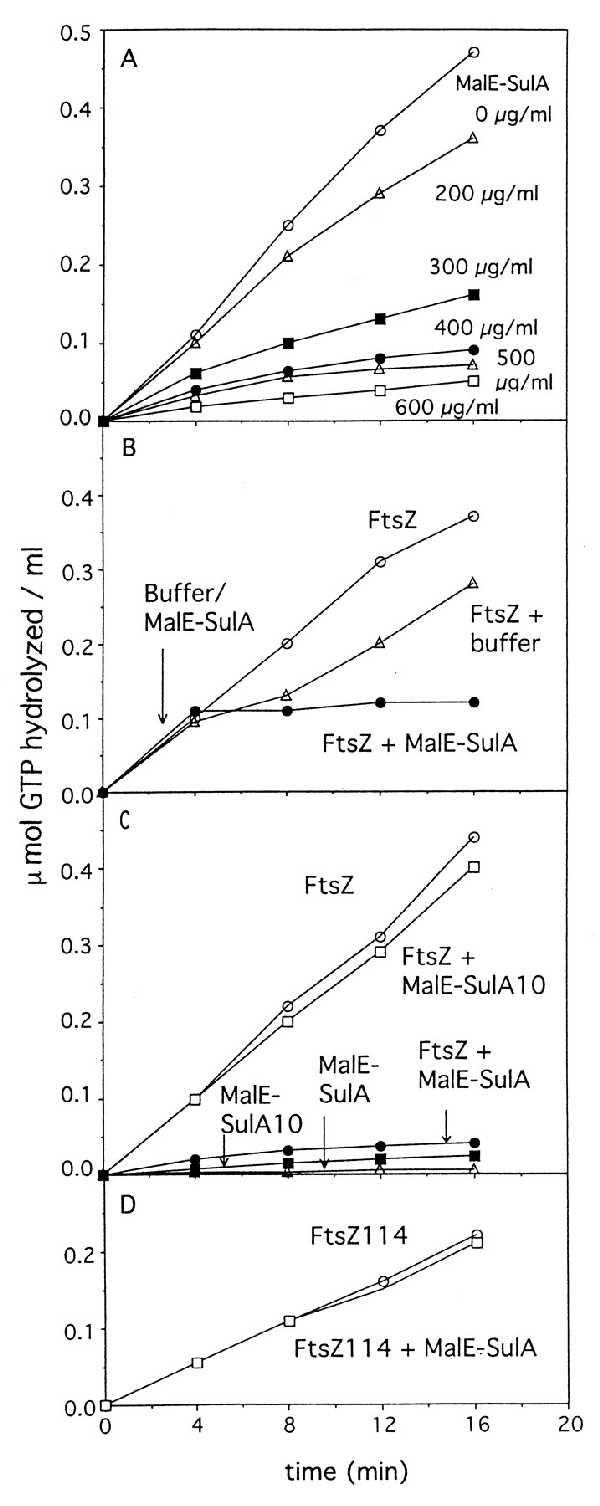
Effect of SulA on the GTPase activity of FtsZ. The GTPase activity was measured in the following buffer: 50 mM Mes–NaOH, pH 6.5/10 mM MgCl2/200 mM KCl. The reaction was initiated with the addition of GTP and incubation at 30°C. At various times samples were removed and GTP hydrolysis was measured by the amount of Pi released. (A) MalE-SulA inhibits the FtsZ GTPase activity. The FtsZ concentration in the reaction was 160 μg/ml and the amount of MalE-SulA is indicated. (B) MalE-SulA immediately inhibits the FtsZ GTPase activity. The GTPase reaction was initiated and divided into two parts; to one part the MalE-SulA fusion was added and to the other buffer was added at the indicated time. (C) MalE-SulA10 does not inhibit the GTPase activity of FtsZ. The MalE-SulA10 mutant protein was added at zero time to a concentration of 600 μg/ml. The FtsZ concentration was 160 μg/ml. (D) Effect of MalE-SulA fusion on the GTPase activity of FtsZ114. The GTPase reaction was carried out with 160 μg/ml FtsZ114 and 600 μg/ml the MalE-SulA fusion.
We next examined the effect of adding MalE-SulA to a reaction in which the GTPase had been initiated (Fig. 2B). In this experiment the GTPase reaction was initiated at time zero and at 4 min either buffer or the fusion protein was added. The additions reduced the FtsZ concentration from 160 μg/ml to 120 μg/ml and so the buffer addition alone slightly decreased the GTPase activity. However, the addition of the MalE-SulA fusion (600 μg/ml) led to an immediate inhibition of the GTPase activity. Thus, the MalE-SulA fusion can rapidly inhibit the GTPase activity of FtsZ.
To further test the specificity of this inhibition we examined the effect of one of the mutant protein fusions, MalE-SulA10 (Fig. 2C). This fusion was purified in identical fashion to the wild-type fusion and was found to have no effect on FtsZ GTPase activity even at a molar ratio of 2.5:1. Thus, the inability of this protein to inhibit division in vivo coincides with an inability of this protein to inhibit the GTPase activity of FtsZ in vitro.
We next examined the effect of the MalE-SulA fusion on FtsZ114. ftsZ114 was isolated as a mutation conferring resistance to SulA, and cells carrying this mutation grow and divide normally even when SulA is induced (23). The GTPase activity of FtsZ114 has been previously characterized and has been shown to have a reduced activity, approximately 50% of the wild-type activity (31). The effect of the MalE-SulA fusion was examined in a reaction in which the molar ratio of the fusion to FtsZ114 was 2.5:1. Although this ratio inhibited wild-type FtsZ activity greater than 90% it had no effect on FtsZ114 (Fig. 2D). Thus, the GTPase activity of FtsZ114 is resistant to SulA.
Effect of MalE-SulA on the Polymerization of FtsZ.
To test the effect of MalE-SulA on the polymerization of FtsZ we used a procedure for FtsZ polymerization that does not require any promoting agents for polymerization. Under these conditions the polymerization is strictly GTP dependent and can be readily assayed by negative-stain electron microscopy or sedimentation (16).
The effect of the MalE-SulA fusion on the polymerization of FtsZ was determined by mixing FtsZ (160 μg/ml) with different concentrations of the MalE-SulA fusion in polymerizing buffer (50 mM Mes, pH 6.5/10 mM MgCl2/200 mM KCl). Polymerization was initiated by adding 1 mM GTP and incubating at 30°C. At 10 min samples were taken and examined by negative-stain electron microscopy (Fig. 3). With the addition of the MalE-SulA fusion at 300 μg/ml, FtsZ polymers were not readily detectable. Adding the fusion at 200 μg/ml also had a significant effect, as polymers were only sporadically observed and these were generally short. At 100 μg/ml the inhibition was much less dramatic, although the quantity and length of the polymers were visibly decreased. Thus, the MalE-SulA fusion inhibited the polymerization of FtsZ in a dose-dependent manner.
Figure 3.
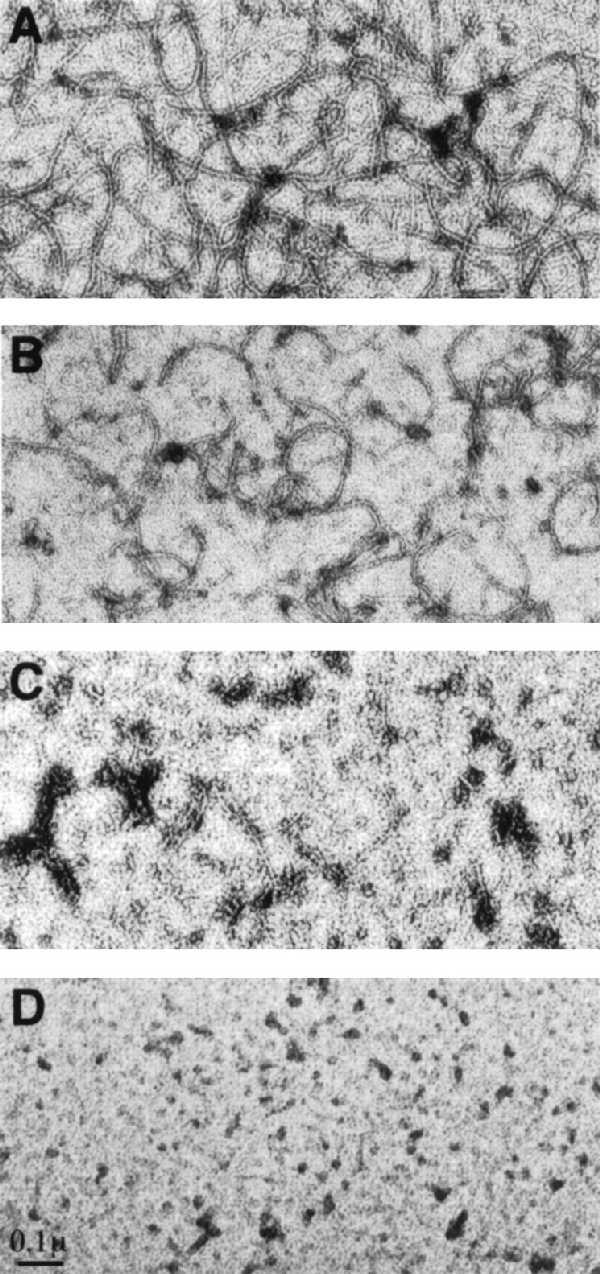
The MalE-SulA fusion inhibits polymerization of FtsZ. FtsZ at 160 μg/ml was diluted into polymerization buffer along with different concentrations of the MalE-SulA fusion. GTP was added to 1 mM and the samples were incubated at 30°C. At 10 min samples were taken and examined by negative-stain electron microscopy. The concentration of the MalE-SulA fusion was 0, 100, 200, and 300 μg/ml in A, B, C, and D, respectively.
We next determined the effect of the MalE-SulA fusion on preexisting polymers. In this experiment the concentration of KCl was 50 mM and polymerization of FtsZ (160 μg/ml) was initiated by the addition of 1 mM GTP and shifting to 30°C. Ten minutes later the MalE-SulA fusion was added at 200 μg/ml, a concentration that is sufficient to prevent polymerization when added at the start, and incubation was continued for 5 min. Samples were then taken for electron microscopy. We observed that 5 min after addition of the fusion the polymers had disappeared (Fig. 4B). In contrast, the filaments were still observed 5 min after the MalE-SulA10 fusion was added at 200 μg/ml (Fig. 4C).
Figure 4.
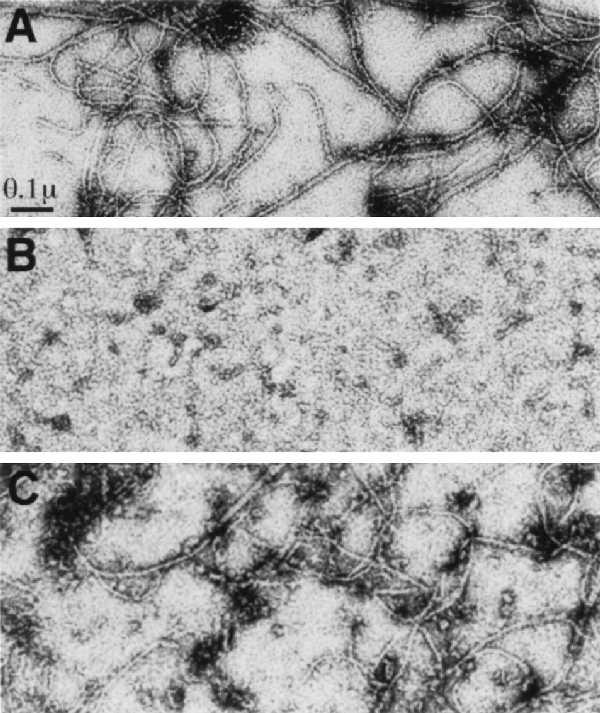
The MalE-SulA fusion promotes depolymerization of FtsZ polymers. The polymerization of FtsZ (160 μg/ml) was initiated in polymerizing buffer containing 50 mM KCl by the addition of 1 mM GTP and incubating at 30°C. Ten minutes later buffer (A), MalE-SulA at 200 μg/ml (B), or MalE-SulA10 at 200 μg/ml (C) was added. The additions resulted in less than a 10% dilution. After an additional 5-min incubation all samples were examined by negative-stain electron microscopy.
The sedimentation assay was also used to determine the effect of the MalE-SulA fusions on polymerization and to compare this to the effect of MalE-SulA10. FtsZ at 160 μg/ml was mixed with different concentrations of the fusion, and polymerization was initiated with the addition of 1 mM GTP and incubation at 25°C. The samples were centrifuged and the amount of FtsZ in the pellet was analyzed by SDS/PAGE. As the concentration of the MalE-SulA fusion was increased (from 0 to 300 μg/ml) we observed a decrease in the FtsZ in the pellet, indicating that the fusion was inhibiting polymerization (Fig. 5). This inhibition parallels the inhibition observed by electron microscopy (Fig. 3). As a control we examined the effect of the MalE-SulA10 fusion. Fig. 5 shows that the MalE-SulA10 did not inhibit polymerization of FtsZ over same concentration range in which the wild-type SulA fusion dramatically inhibited polymerization. Furthermore, using this assay, we observed that FtsZ114 polymerizes to an extent similar to that of wild type and is not inhibited by MalE-SulA (data not shown).
Figure 5.

Comparison of the effects of MalE-SulA and MalE-SulA10 on FtsZ polymerization. The effects of MalE-SulA fusions were determined by a sedimentation assay. FtsZ was diluted to 160 μg/ml in polymerizing buffer containing 50 mM KCl in the presence of increasing concentrations of MalE-SulA or MalE-SulA10 as indicated. After the addition of 1 mM GTP, samples were centrifuged for 10 min at 80,000 rpm in a Beckman TL-100 centrifuge. The amount of FtsZ in the pellet was visualized by SDS/PAGE and Coomassie staining and quantitated with a Molecular Dynamics densitometer. With GTP 50% of the total FtsZ was recovered in the pellet as observed earlier (16). With the addition of MalE-SulA at 50, 100, and 200 μg/ml the FtsZ recovered was 39%, 20%, and 12%, respectively. With the addition of MalE-SulA10 at 50, 100, and 200 μg/ml the FtsZ recovered was 36%, 37%, and 42%, respectively. This slight reduction in FtsZ recovery observed with MalE-SulA10 was also observed with MalE alone, indicating it was a nonspecific effect.
DISCUSSION
Previous work has shown that induction of SulA, a well characterized inhibitor of division in E. coli, blocks formation of the Z ring, thereby preventing cell division (27). In this study we show that a MalE-SulA fusion that retains the ability to block formation of the Z ring in vivo blocks the GTPase activity and polymerization of FtsZ in vitro. This ability of SulA to inhibit polymerization of FtsZ in vitro provides a mechanism by which it could prevent formation of the Z ring in vivo. Accordingly, after DNA damage SulA is induced as part of the SOS response it binds to FtsZ, preventing polymerization and formation of the Z ring. In addition, SulA causes preformed Z rings to disappear, thereby quickly inhibiting division. Upon repair of DNA, SulA induction ceases as the SOS response is shut off, and SulA is rapidly removed by proteolysis. The removal of SulA restores the ability of FtsZ to polymerize and reform Z rings.
Our results show that the MalE-SulA fusion inhibits the GTPase activity and polymerization of FtsZ. At a molar ratio of about 1 we found that this fusion inhibits the GTPase activity ≈50% and markedly inhibits FtsZ polymerization. This inhibitory activity of MalE-SulA correlates with its ability to block Z ring formation in vivo. In contrast to these results, the MalE-SulA10 fusion did not inhibit the GTPase activity or polymerization. The inability of MalE-SulA10 to inhibit these activities of FtsZ correlates with its inability to inhibit Z ring formation in vivo. In addition to the sulA mutations we also examined the ftsZ114 mutation, which makes cells refractory to SulA inhibition (35). Consistent with our previous findings, which showed that the ftsZ114 mutation eliminated the interaction between FtsZ and SulA (28), we found that the GTPase activity and polymerization of FtsZ114 were not inhibited by MalE-SulA.
Previously Higashitani et al. (29) reported that a MalE-SulA fusion, similar to the one constructed here, interacted with FtsZ. They found that the interaction required GTP hydrolysis but that the fusion had no effect on GTP hydrolysis. We cannot reconcile their result, since we observe that MalE-SulA dramatically inhibits the GTPase activity. They also reported a stoichiometric complex between FtsZ and MalE-SulA under GTP hydrolysis conditions. We find that FtsZ is only weakly retained on a MalE-SulA affinity column and that the retention is independent of GTP (data not shown).
How does SulA prevent the GTPase activity and polymerization of FtsZ? Previously, we showed that SulA and FtsZ interact directly in the yeast two-hybrid system (28). Also, it has been suggested that the GTPase activity of FtsZ depends upon subunit interaction (7), and it is known that the dynamic behavior of the polymers depends upon the GTPase activity (16). Therefore, it is likely that SulA binds to FtsZ, preventing interaction between subunits that would lead to the GTPase activity. Interestingly, SulA does not appear to mask a site on FtsZ involved directly in subunit interaction because FtsZ2, which still binds SulA, can function in the presence of SulA (31, 35). SulA may function similarly to the tubulin-binding protein strathmin that sequesters tubulin dimers (36). Such action by SulA would also explains its ability to cause disassembly of FtsZ polymers. By sequestering FtsZ subunits polymer growth is prevented and preformed polymers, unable to grow, readily disassemble.
Although SulA is an efficient inhibitor of cell division, cell division is still blocked in the absence of SulA, indicating that there is at least one other mechanism that can block septation (37–39). Recent evidence suggests that an additional inhibitor is produced in response to DNA damage, but unlike SulA, may have to undergo posttranslational activation (39). Additionally, some step in DNA replication could act to promote Z ring formation, and this step would be blocked by inhibiting replication.
The model for formation of the Z ring includes a nucleation event followed by polymerization (2, 3). Our recent demonstration that FtsZ polymers are dynamic due to GTP hydrolysis provides a basis for the dynamic localization of FtsZ and supports the model for Z ring formation (16). Our present results demonstrate that a well recognized inhibitor of division, SulA, produced in response to DNA damage, blocks FtsZ polymerization, providing a mechanism by which it blocks Z ring formation.
Acknowledgments
We thank Barbara Fegley for assistance with the electron microscopy. This work was supported by Public Health Service Grant GM 29764 from the National Institutes of Health.
References
- 1.Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffe A. J Bacteriol. 1989;171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi E, Lutkenhaus J. Nature (London) 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 3.Lutkenhaus J. Mol Microbiol. 1993;9:404–409. doi: 10.1111/j.1365-2958.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 4.Addinall S G, Bi E, Lutkenhaus J. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pogliano J, Pogliano K, Weiss D S, Losick R, Beckwith J. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addinall S G, Lutkenhaus J. Mol Microbiol. 1996;22:231–238. doi: 10.1046/j.1365-2958.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- 7.Lutkenhaus J, Addinall S G. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Ehrhardt D W, Margolin W. Proc Natl Acad Sci USA. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addinall S G, Cao C, Lutkenhaus J. Mol Microbiol. 1997;25:303–310. doi: 10.1046/j.1365-2958.1997.4641833.x. [DOI] [PubMed] [Google Scholar]
- 10.RayChaudhuri D, Park J T. Nature (London) 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A, Lutkenhaus J. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson H P. Cell. 1995;80:367–370. doi: 10.1016/0092-8674(95)90486-7. [DOI] [PubMed] [Google Scholar]
- 13.de Boer P, Crossley R, Rothfield L. Nature (London) 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee A, Dai K, Lutkenhaus J. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson H P, Taylor D W, Taylor K A, Bramhill D. Proc Natl Acad Sci USA. 1996;93:519–523. doi: 10.1073/pnas.93.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Lutkenhaus J. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker G C. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1400–1416. [Google Scholar]
- 18.Huisman O, D’Ari R. Nature (London) 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- 19.Mitzusawa S, Gottesman S. Proc Natl Acad Sci USA. 1983;80:358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguin E, Lutkenhaus J, D’Ari R. J Bacteriol. 1986;166:733–738. doi: 10.1128/jb.166.3.733-738.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottesman S, Halpern E, Trisler P. J Bacteriol. 1981;148:265–273. doi: 10.1128/jb.148.1.265-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutkenhaus J. J Bacteriol. 1983;154:1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George J, Castellazzi M, Butin G. Mol Gen Genet. 1975;140:309–332. [PubMed] [Google Scholar]
- 24.Johnson B F. Genet Res. 1977;30:273–286. doi: 10.1017/s0016672300017687. [DOI] [PubMed] [Google Scholar]
- 25.Gayda R C, Yamamoto L T, Markovitz A. J Bacteriol. 1976;127:1208–1216. doi: 10.1128/jb.127.3.1208-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutkenhaus J, Sanjanwala B, Lowe M. J Bacteriol. 1986;166:756–762. doi: 10.1128/jb.166.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi E, Lutkenhaus J. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Cao C, Lutkenhaus J. J Bacteriol. 1996;178:5080–5085. doi: 10.1128/jb.178.17.5080-5085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashitani A, Higashitani N, Horiuchi K. Biochem Biophys Res Commun. 1995;209:198–204. doi: 10.1006/bbrc.1995.1489. [DOI] [PubMed] [Google Scholar]
- 30.RayChaudhuri D, Park J T. J Biol Chem. 1994;269:22941–22944. [PubMed] [Google Scholar]
- 31.Dai K, Mukherjee A, Xu Y, Lutkenhaus J. J Bacteriol. 1994;176:130–136. doi: 10.1128/jb.176.1.130-136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzman L-Z, Belin D, Carson M J, Beckwith J. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai K, Xu Y, Lutkenhaus J. J Bacteriol. 1996;178:1328–1334. doi: 10.1128/jb.178.5.1328-1334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field C M, Al-Awar O, Rosenblatt J, Wong M L, Alberts B. J Cell Biol. 1996;133:605–616. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi E, Lutkenhaus J. J Bacteriol. 1990;172:5602–5609. doi: 10.1128/jb.172.10.5602-5609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jourdain L, Curmi P, Sobel A, Pantaloni D, Carlier M-F. Biochemistry. 1997;36:10817–10821. doi: 10.1021/bi971491b. [DOI] [PubMed] [Google Scholar]
- 37.Burton P, Holland I B. Mol Gen Genet. 1983;190:128–132. doi: 10.1007/BF00330334. [DOI] [PubMed] [Google Scholar]
- 38.Bork P, Sander C, Valencia A. Proc Natl Acad Sci USA. 1994;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill T M, Sharma B, Valjavec-Gratian M, Smith J. J Bacteriol. 1997;179:1931–1939. doi: 10.1128/jb.179.6.1931-1939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


