Abstract
The present study used two versions of a spatial list learning (SLL) paradigm to examine the effects of increased cognitive load on visuospatial working memory processes in young and old beagle dogs. In the first experiment, young, and a select group of old dogs were first presented with one item, then two, and then three, and were rewarded for responding to the novel position. The dogs were able to learn the task at short delays, but compared with young dogs, old dogs performed worse at delays of 10 sec, and could not reach longer delays. Analysis of errors indicated that memory was best for end items in the spatial list and that within sessions, the number of errors in later trials was greater than the number of errors in earlier trials. A second version of the task, a modified SLL (mSLL) was developed to control for the use of non-mnemonic strategies on the SLL task. In this version, the first two items were presented individually. Acquisition and maximal memory performance were better in the young relative to the old dogs. Similar to the original SLL design, memory for early list items was worse than memory for later list items in both young and old dogs. The within-session pattern of errors however, did not change from trial to trial on the mSLL. The present results suggest that multiple working memory processes are engaged during complex tests of visuospatial function and the neuroanatomical substrates controlling these processes are affected differentially by age in the beagle dog.
Visuospatial functioning involves the ability to perceive, remember, and manipulate visual information within a spatial context. Visuospatial function impairments occur during normal aging (Botwinick 1984; Mazaux et al. 1995; Harvey and Mohs 2001), but are particularly prevalent in conditions of dementia (Freedman and Oscar-Berman 1989; Flicker et al. 1991; Harvey and Mohs 2001). In many patients with Alzheimer's disease, visuospatial deficits are the most prominent behavioral symptom to appear early in the course of the disease (Martin 1987; Becker et al. 1988; Nebes 1992) and account for impairments in complex discriminations of faces (Eslinger and Benton 1983), patterns (Brouwers et al. 1984), random shapes (Huff et al. 1987), and geometric arrays (Becker et al. 1988; Mendez et al. 1990).
Our research program has focused primarily on developing a canine model of age-dependent cognitive decline using the beagle dog (Cummings et al. 1996; Adams et al. 2000a,b; Callahan et al. 2000; Head et al. 2001; Chan et al. 2002), and a major area of research has included tests of visuospatial ability as a function of age (Head et al. 1995, 1998; Milgram et al. 1999, 2002; Adams et al. 2000b; Chan et al. 2002). Thus far, we have developed two distinct protocols. The first is based on acquisition of a landmark discrimination task, as described originally by Pohl (1973), and the second uses a delayed nonmatching to position (DNMP) procedure. The landmark task examines allocentric visuo-spatial ability, which is based on a subject's use of the location of a defined landmark to determine the correct target response (Milgram et al. 1999). In the landmark task, dogs are presented with two identical discriminanda, distinguishable only by their proximity to a thin yellow rod, which serves as the landmark. Only correct responses to the discriminanda closest to the landmark object are rewarded. In both young and old dogs, performance on this task declines as the distance between the landmark and the rewarded discriminandum increases. Compared with old dogs, however, young dogs typically learn the task faster and are better at solving the task when the distance between the rewarded discriminandum and landmark increases (Milgram et al. 2000; Estrada et al. 2001).
The DNMP paradigm provides an index of both visuospatial learning and working memory (Adams 2000a,b; Chan et al. 2002). In the original design of this task, the 2-component DNMP (2cDNMP), dogs are presented with a sample stimulus at one of two spatial locations (Head et al. 1995). After a delay, the sample appears in the original location paired with a second identical stimulus in the remaining location. To obtain a food reward, the dog must choose the novel stimulus location. This task was later modified to a 3-component DNMP (3cDNMP) to minimize the use of non-mnemonic orienting strategies adopted by some of the dogs on the 2cDNMP (Chan et al. 2002). For the 3cDNMP, the sample stimulus appears in one of three potential locations and is followed by an identical stimulus placed in one of the two remaining positions after a fixed delay. Only responses to the novel, non-match location are rewarded. Working memory demands on both tasks are varied by changing the delay interval between the sample and non-match positions, which determines the amount of time that the spatial information must be maintained in working memory. Typically, all dogs show a decline in accuracy when the working memory demands are increased with longer delay intervals. Most young dogs show a very small performance decrement with increasing delays. The variability in performance is much greater among the old dogs. Some old dogs are impaired in learning the task at short delays, but once acquired, can remember spatial information as well as young dogs at long delays. The opposite can also occur when aged dogs learn the task as quickly as young dogs, but cannot attain the same accuracy as the demands of the task (i.e., delay intervals) increase (Adams et al. 2000b; Chan et al. 2002).
Studies of human working memory indicate that tasks that place greater demands on working memory show greater age sensitivity than tasks that place weaker demands on working memory (Gick et al. 1988; Dobbs and Rule 1989; Salthouse et al. 1989; Babcock and Salthouse 1990; Craik et al. 1990; Salthouse and Babcock 1991; Craik and Jennings 1992; Salthouse 1992, 1993; Frieske and Park 1993; Morrell and Park 1993). Increasing the amount of time that information must be maintained in working memory is one way to increase the demands on working memory processes. A second is by increasing the amount of information that must be maintained and monitored during a task. Modifying the amount of information that must be retained in working memory is accomplished with list learning tasks. These typically involve presenting the subjects with a serial array of categorically defined stimuli (e.g., objects, digits, or words) and testing memory for the items following a brief delay. Thus, the longer the list, the greater the demands on working memory. When the delay between the list presentation and memory test is sufficiently long, a characteristic U-shaped, or serial-position function emerges, in which items at the beginning and end of the list are remembered better than items in the middle of the list (Ebbinghaus 1902, 1964; Raffel 1936; Welch and Burnett 1942; Baddeley 1986, 2001;). These effects, called primacy-recency effects, occur in humans (Crowder 1976; Sands and Wright 1980a,b; Roberts and Kraemer 1981; Wright et al. 1985; Korsnes and Magnussen 1996), nonhuman primates (Sands and Wright 1980a,b; Buchanan et al. 1981; Roberts and Kraemer 1981; Wright et al. 1984, 1985; Castro and Larsen 1992; Castro 1995, 1997; Wright and Rivera 1997; Wright 1998, 1999), dolphins (Thompson and Herman 1977), pigeons (Santiago and Wright 1984; Terrace et al. 1995), and rats (Roberts and Smythe 1979; Kesner and Novak 1982; DiMattia and Kesner 1984; Kesner et al. 1984; Kesner and Holbrook 1987; Bohluis and van Kampen 1988; Deacon and Rawlins 1995).
The present study describes the development of a spatial-based list learning (SLL) task to examine the effect of age on the performance of beagle dogs. This task, a modification of our 3c DNMP task (Chan et al. 2002), involves the presentation of identical objects at multiple spatial locations that must be maintained in memory during delays of increasing length. Compared with our previous DNMP tasks, in which working memory demands were increased by using longer retention intervals, memory demands in the SLL task are altered by increasing both the number of items and the length of time that these items must be retained in working memory prior to the memory test. The task is similar conceptually to a spatial-delayed recognition span task (DRST) used for testing memory span in nonhuman primates (Beason et al. 1990; Herndon et al. 1997; Moss et al. 1997; Beason-Held et al. 1999). Age comparisons on the DRST have indicated that old monkeys are impaired relative to young monkeys (Herndon et al. 1993; Moss et al. 1997; Lacreuse et al. 1999). Accordingly, we designed the SLL task to compare list learning in young and old beagle dogs using various interstimulus retention intervals. In the first experiment, a three-phase non-matching paradigm was used to present an array of spatial stimuli. At each phase, the previous spatial item in the list was presented with a novel spatial location, and only responses to the new location were rewarded. The second experiment involved a modification of the SLL task. In the modified version, a three-phase procedure was also used to present the spatial array, but items were presented individually rather than in pairs.
RESULTS
Experiment I
Pretraining
One aged dog (female) was unable to complete the 3cDNMP task within the maximum 40 d of pretraining, and was therefore dropped from the study. When this animal was excluded from the analysis, the number of errors (U = 12.5, P = .377) and trials (U = 11.00, P = .222) to criterion to complete the pretraining in young and old dogs did not differ significantly.
Spatial List Learning
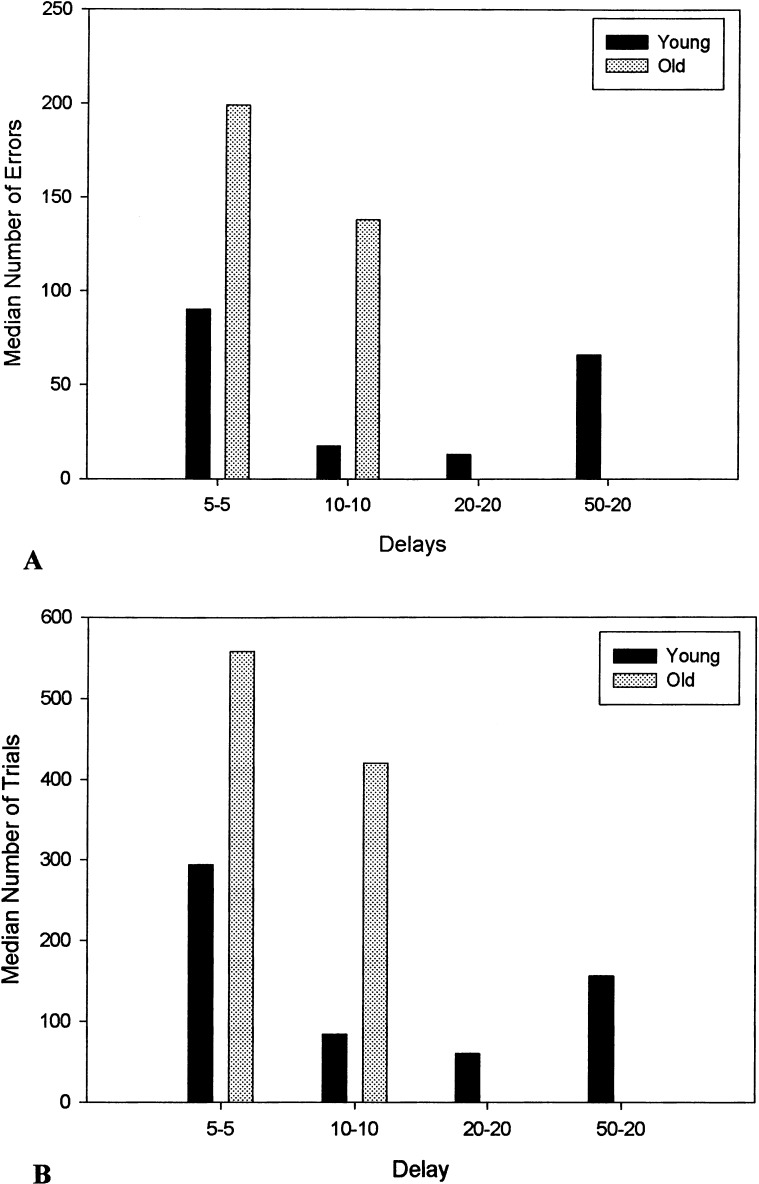
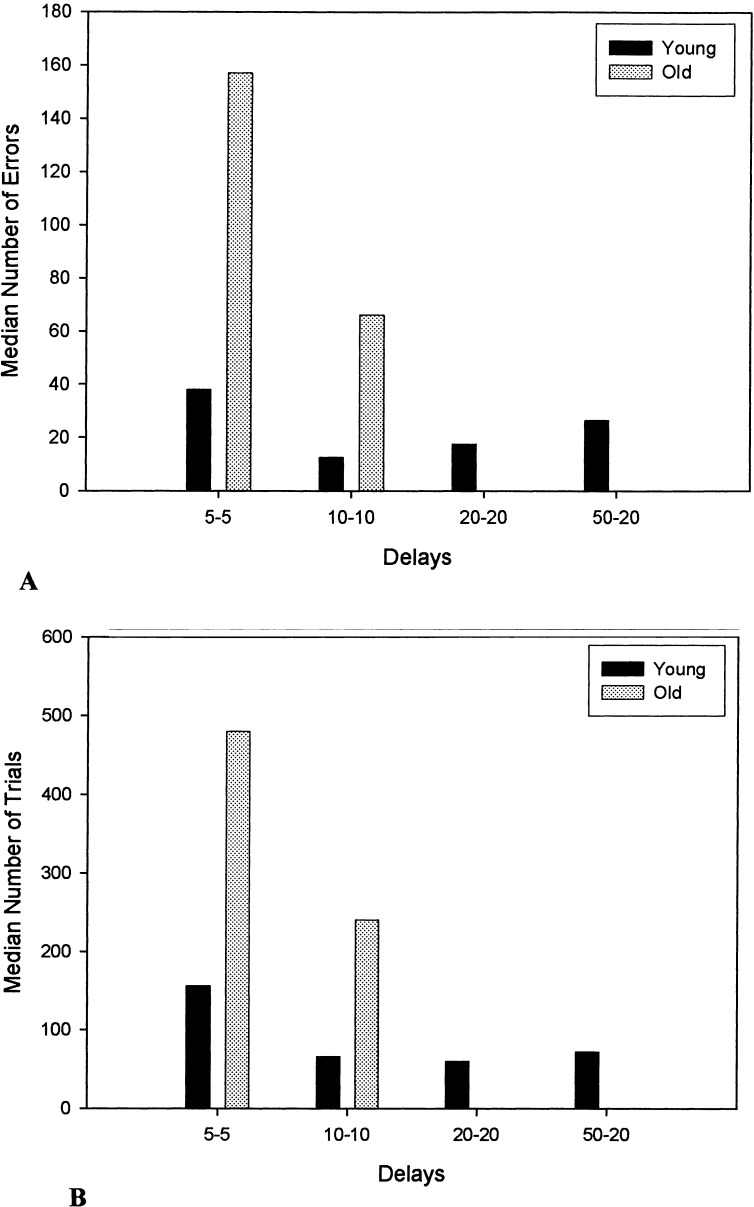
Total number of errors and trials to criterion as well as total primacy and recency errors during the acquisition phase (5-sec delay) of the SLL task for each dog is shown in Table 1. Inspection of this table suggests that the aged females solved the acquisition phase of the task faster than the aged males. In fact, the only two old dogs to complete the task beyond the 5-sec delay were both females. Among these two dogs, one dog successfully reached criterion at 10 sec, but was unable to reach criterion at the 20-sec delay. In contrast, all young dogs reached criterion at 10 sec, and half of these animals (three of six) reached criterion at the 50–20-sec delay. Median number of errors and trials to criterion to complete the SLL task at each delay is shown in Figure 1. Although the young and old dogs did not differ in the number of trials required to solve the task at the shortest delay (U = 8.00, P = .108), the number of errors made by the old dogs was marginally higher than the number of errors made by the young dogs (U = 6.50, P = .065). Compared with the old dogs, the young dogs achieved higher maximal memory scores by completing this task at longer delays (U = 4.50, P = .026).
Table 1.
Performance on the Spatial List Learning Task During the Acquisition Phasea
| Subject | Age (years) | Gender | Errors | Trials | Primacy errors | Recency errors |
|---|---|---|---|---|---|---|
| Old Dogs | ||||||
| Timmy | 14.22 | Male | 280 | 600 | 200 | 80 |
| Henry | 14.19 | Male | 220 | 600 | 179 | 41 |
| Odo | 13.18 | Male | 178 | 468 | 134 | 36 |
| Quark | 12.49 | Male | 267 | 600 | 201 | 66 |
| Piggy | 12.41 | Female | 168 | 480 | 127 | 41 |
| Alicia | 10.13 | Female | 56 | 156 | 35 | 22 |
| Young Dogs | ||||||
| Verne | 3.40 | Male | 15 | 60 | 14 | 1 |
| Carmella | 3.73 | Female | 178 | 540 | 140 | 38 |
| Tomi | 4.31 | Male | 206 | 540 | 173 | 33 |
| Orville | 4.33 | Male | 54 | 192 | 36 | 18 |
| Fuzz | 4.76 | Male | 15 | 72 | 12 | 3 |
| Paul | 6.70 | Male | 127 | 396 | 73 | 48 |
Scores illustrate total errors, trials, and primacy and recency errors to reach criterion for each of the subjects tested at the 5-sec delay.
Figure 1.
Median number of errors (A) and trials (B) to criterion for each delay of the SLL task for the young and old dogs. No values are shown for the old dogs at delays of 20–20 and 50–20, because only young dogs achieved these longer delays.
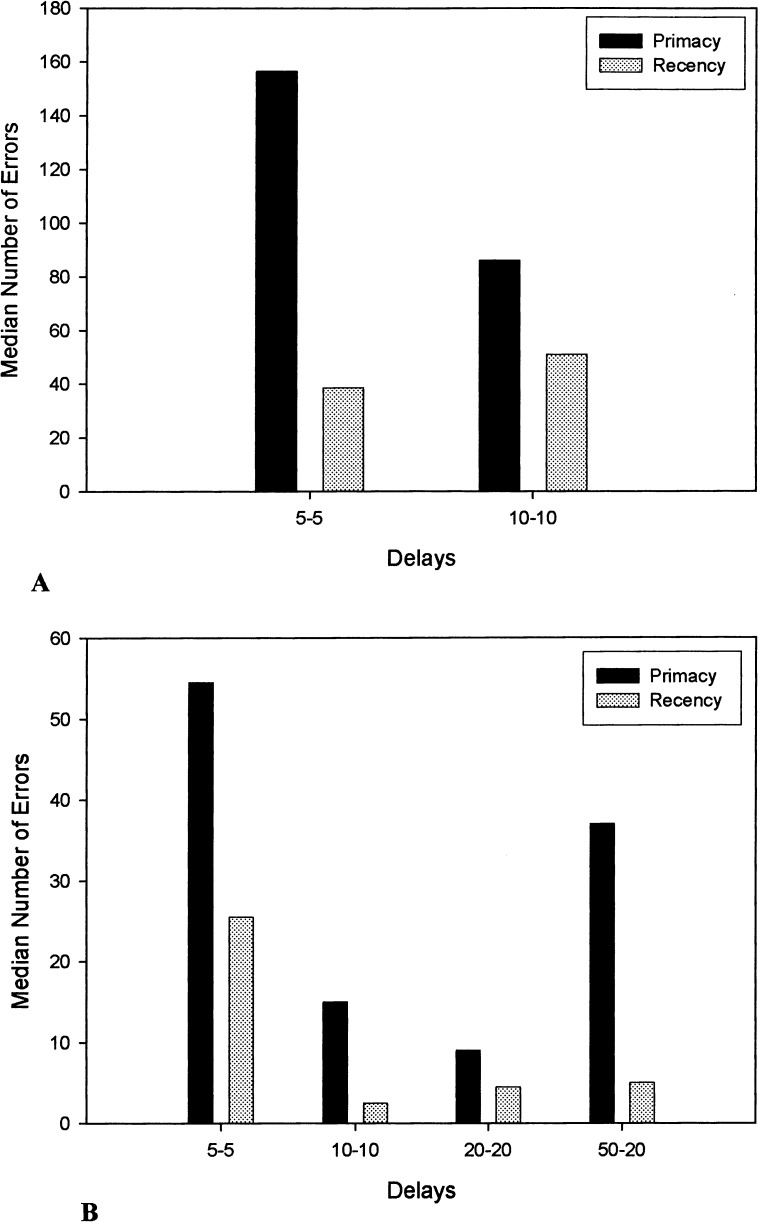
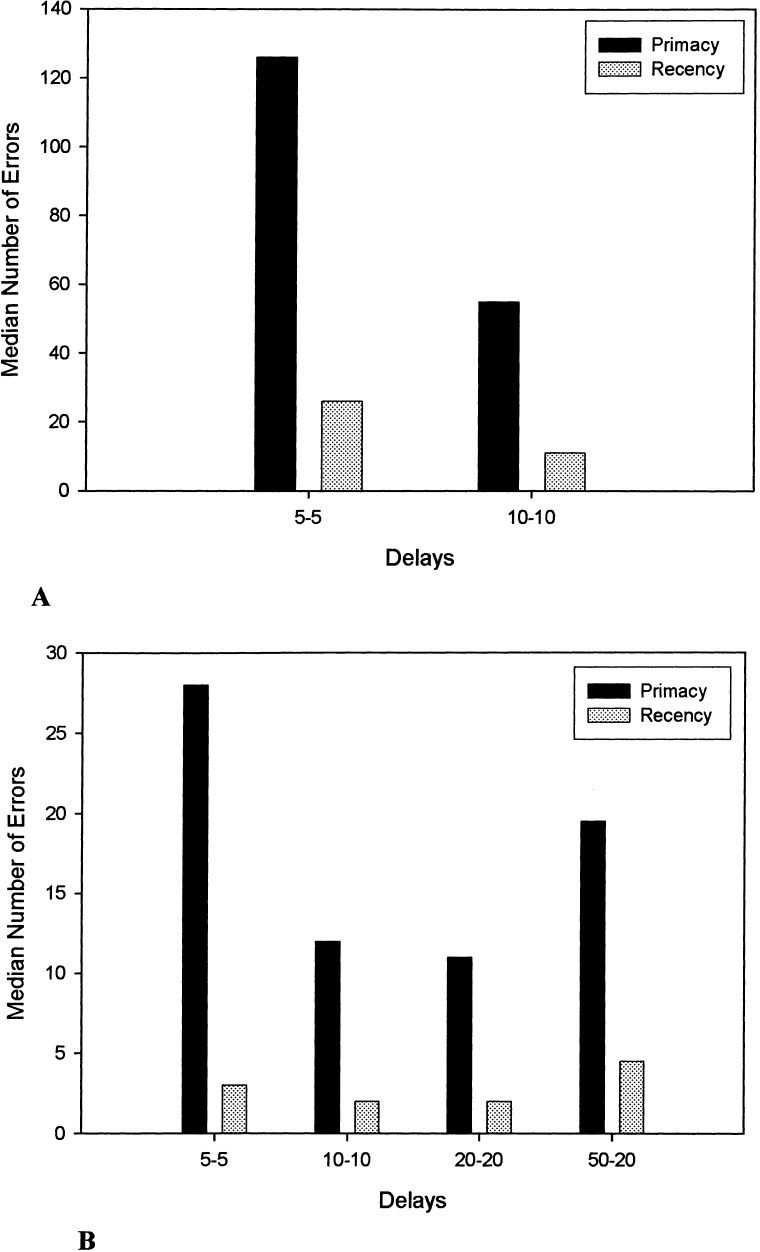
Both groups made more primacy errors than recency errors (Fig. 2). A Sign test analysis indicated that the number of primacy and recency errors in both groups significantly differed from chance (P = .0001). Overall, the number of primacy errors did not differ significantly between the two age groups (U = 8.00, P = .109), but the number of recency errors in the young dogs was marginally lower relative to the old dogs (U = 7.00, P = .078). A within-group comparison of the ratio of primacy-recency errors in the young dogs using a Kruskal-Wallis test indicated that the ratio of primacy-recency errors did not significantly differ as a function of delay interval [H(3) = 1.65, P = .649]. This analysis could not be performed for the old dogs, because only one animal in this group was able to pass the 10-sec delay.
Figure 2.
Median number of primacy and recency errors for old (A) and young (B) dogs at each delay of the original SLL task. Only one old dog completed criterion measures at the 10-sec delay.
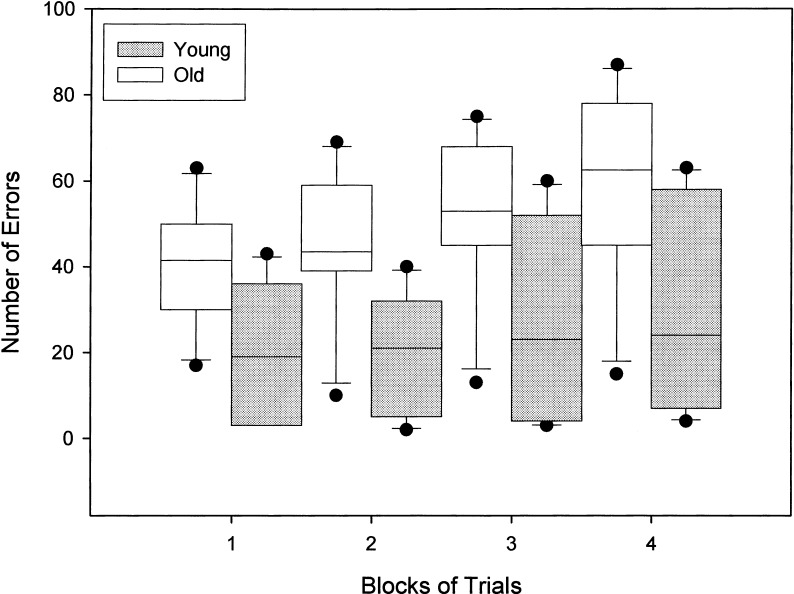
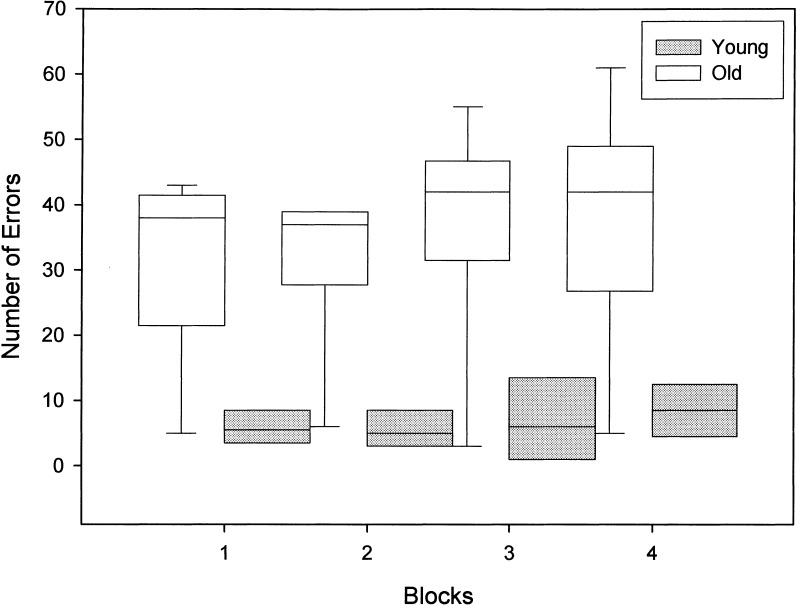
Total number of errors for each block of three trials during SLL acquisition at the 5-sec delay for young and old dogs is shown in Figure 3. A Wilcoxon-Rank Sum test was used to examine the pattern of errors across blocks of trials within each group. For the young dogs, the total number of errors in blocks three (trials 7–9; P = .042) and four (trials 10–12; P = .028) were greater than the number of errors made in block one (trials 1–3). No other comparisons were significant for the young dogs. In the old dogs, the number of errors made during block four were significantly greater than the number of errors made in block one (P = .046), block two (P = .028), and block three (P = .043). The number of errors made in block three were significantly greater than the number of errors committed in block two (P = .027). The old dogs made more errors on block two relative to the young dogs (U = 5.00, P = .037). No other group differences were significant, although the number of errors made on blocks one (U = 7.50, P = .092) and four (U = 7.50, P = .092) were marginally higher for the old dogs compared with the young dogs.
Figure 3.
Box-plot of errors as a function of blocks of trials across all sessions of SLL testing at the 5-sec delay for old and young dogs. Median errors for each block of three trials are indicated by a solid line within the box plot. Longer box plots and whiskers indicate greater variability within each group across blocks of trials. Individual data points represent extreme values.
Conclusion
In the pretraining phase, we found no age differences in acquisition of the DNMP task. Although these results are inconsistent with our earlier findings of age-related impairments on the DNMP task (Adams et al. 2000a,b; Chan et al. 2002), this is likely due to selection factors; young and old aged dogs were trained previously on the DNMP task. The 3cDNMP was used as a screening device. Successful completion of the pretraining measure was essential for inclusion on the more complicated SLL task. One old female dog, who had limited DNMP experience relative to the other old dogs, was unable to pass the DNMP criteria at the shortest delay (i.e., 5 sec), and was withdrawn from the study. Thus, the absence of age effects on the DNMP pretraining was not surprising, as the analysis involved only highly experienced old dogs that completed the prescreening procedures successfully.
Initially, we believed that manipulating both the amount of information and the duration in which this information was stored in memory would increase working memory load and represent a more sensitive measure of age-related working memory impairments in beagle dogs. Surprisingly, young and old dogs did not differ significantly in their acquisition of the SLL task, but two factors may account for the lack of age effects during learning of the SLL task. First, similarities between the SLL and DNMP protocols may have facilitated acquisition in the SLL condition. On each trial of the SLL, the second lid was always presented with the first lid using a non-match protocol, which is identical to the procedures used on DNMP tasks. Because the old dogs were highly experienced with DNMP procedures, this format may have facilitated acquisition of the SLL task. Second, previous studies of DNMP performance in our laboratory indicate that some dogs solve the task using noncognitive strategies. Examination of interstimulus behaviors on the DNMP task led Chan et al. (2002) to conclude that dogs can solve this task by maintaining a fixed posture and orienting toward the correct location during the interstimulus delay. It is therefore possible that similar orienting behaviors were used to solve the SLL task, which was similar to the design of a DNMP procedure. Experiment II was designed to produce a novel SLL procedure distinct from DNMP protocols and to minimize the potential use of noncognitive strategies.
RESULTS
Experiment II
The second experiment used a modified version of the original SLL task to minimize the use of non-mnemonic strategies and maximize the cognitive demands of working memory. This was accomplished by presenting the first and second discriminandum separately, rather than concurrently. The objective of this modification was to improve the sensitivity of the spatial-list task to age-related changes in visuospatial working memory.
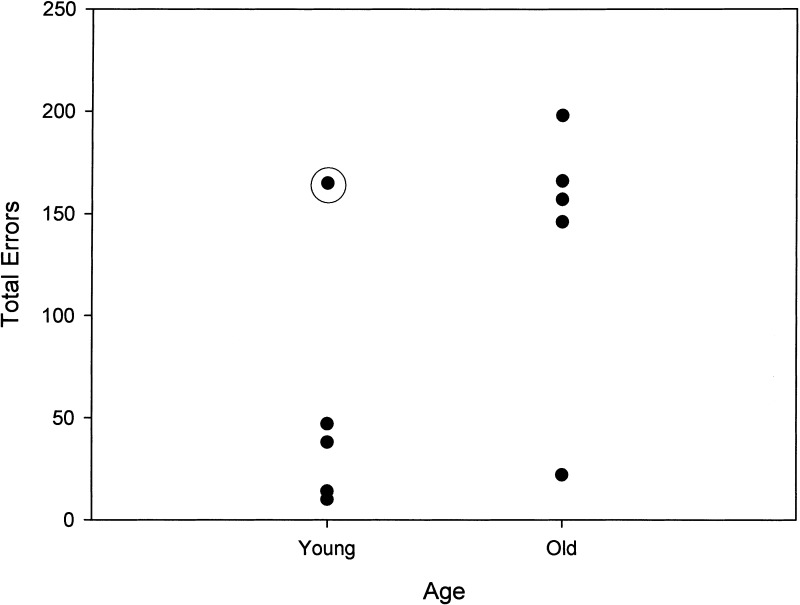
Table 2 displays total errors and trials to criterion as well as primacy and recency scores on the acquisition phase of the task for males and females in the young and old groups. The only old dog to complete the modified task beyond the 5-sec delay was the lone female in the group. This dog, the same animal that reached criterion on the original SLL at 5 sec, was unable to reach criterion at the 10-sec delay on the modified task. In contrast, four of five young dogs reached criterion at 5 sec, and all of these dogs reached criterion at the 50–20-sec delay. One young male dog made more errors on the mSLL task compared with the other young dogs, and was unable to learn the mSLL task at the 5-sec delay (Fig. 4). This dog was more than 2-yr older than the oldest young dog, and was, in fact, closer to middle age. When this animal was removed from the analysis, young dogs made significantly fewer errors (U = 2.00, P = .050) and required fewer trials (U = 2.00, P = .041) to complete the mSLL task compared with the old dogs (Fig. 5). In addition, analysis of maximal memory scores indicated that young dogs reached higher-delay intervals compared with the old dogs (U = 1.50, P = .006).
Table 2.
Performance on the Modified Spatial List Learning Task During the Acquisition Phasea
| Subject | Age (years) | Gender | Errors | Trials | Primacy errors | Recency errors |
|---|---|---|---|---|---|---|
| Old Dogs | ||||||
| Timmy | 14.22 | Male | 166 | 480 | 126 | 40 |
| Henry | 14.19 | Male | 146 | 480 | 124 | 22 |
| Odo | 13.18 | Male | 157 | 480 | 131 | 26 |
| Quark | 12.49 | Male | 198 | 480 | 154 | 44 |
| Piggy | 12.41 | Female | 22 | 96 | 19 | 3 |
| Young Dogs | ||||||
| Verne | 3.40 | Male | 10 | 60 | 8 | 2 |
| Carmella | 3.73 | Female | 14 | 72 | 11 | 3 |
| Tomi | 4.31 | Male | 38 | 156 | 36 | 3 |
| Orville | 4.33 | Male | 47 | 180 | 28 | 19 |
| Paul | 6.70 | Male | 165 | 480 | 121 | 44 |
Scores illustrate total errors, trials, and primacy and recency errors to reach criterion for each of the subjects tested at the 5-sec delay.
Figure 4.
Distribution of total errors on mSLL task. One young male dog (circled) was unable to complete the mSLL task at 5 sec. This young dog was 2-yr older than the oldest young dog and was closer to middle-aged.
Figure 5.
Median number of errors (A) and trials (B) to criterion for each delay of the SLL task for the young and old dogs. No values are shown for the old dogs at delays of 20–20 and 50–20, because only young dogs achieved these longer delays.
The analysis of the pattern of errors between the old and young dogs indicated that, overall, young dogs made fewer total primacy errors (U = 2.00, P = .050) and recency errors (U = 2.00, P = .046) relative to the old dogs (Fig. 6). A Sign test analysis indicated that the number of primacy and recency errors in both groups differed significantly from chance (P = .002). A Kruskal-Wallis test of the ratio of primacy-recency errors within the young dogs, however, did not differ significantly across the delay intervals [H(3) = 1.26, P = .739]. Only one old dog completed mSLL testing at delays longer than 5 sec, so the ratio of primacy-recency errors beyond 5 sec could not be assessed for the old dogs.
Figure 6.
Median number of primacy and recency errors for old (A) and young (B) dogs on the mSLL task. Errors at the 10-sec delay represent a single old dog. No other old dog completed the 10-sec delay.
Total number of errors for each block of three trials during SLL acquisition at the 5-sec delay for young and old dogs is shown in Figure 7. Overall, the old dogs made more errors on block one (U = 2.00, P = .050), two (U = 2.00, P = .048), and three (U = 2.00, P = .049) compared with the young dogs. The number of errors on block four was only marginally higher in the old dogs (U = 3.00, P = .086). Within the young and old dog groups, however, the number of total errors did not differ significantly between blocks of trials (P > .05)
Figure 7.
Box-plot of errors as a function of blocks of three trials across all sessions of mSLL testing at the 5-sec delay for old and young dogs. Median errors for each block of three trials are indicated by a solid line within the box plot. Longer box-plots and whiskers indicate greater variability within each group across blocks of trials.
Conclusion
The mSLL task proved to be more difficult for the aged dogs than the young, producing significant age effects. Compared with the young dogs, who achieved higher maximal memory scores, the old dogs made significantly more errors and required more trials to reach criterion at the shortest delay, and as a group, were unable to solve the task at delays greater than 5 sec. Modifying the SLL task also changed the distribution of errors. The trend for more errors to occur during later trials within each session, which was observed on the original task, disappeared with the mSLL procedure. Overall, old dogs made more errors on each block of three trials relative to the young dogs. The total number of primacy and recency errors in the young dogs was significantly lower relative to the old dogs, but primacy errors still outnumbered recency errors in both groups.
DISCUSSION
The present study describes two different versions of a spatial list task, which were developed to examine the effect of age on complex visuospatial learning and memory in beagle dogs. The original SLL task was clearly more difficult than the 3cDNMP task. Among the old dogs, the only two dogs to complete the SLL task at the 5-sec delay were both female, and no old dog completed the task beyond 10 sec. All young dogs, in contrast, reached criterion at 20 sec, and more than half of these dogs completed the task at the highest delay (i.e., 50 sec).
The protocol used for the SLL task is similar to DNMP paradigms, because two cognitive processes are essential for accurate performance as follows: (1) The animal must acquire the basic procedural rule of responding to nonmatched locations, and (2) within a trial, the animal must remember the location of a previous object and respond to the novel location. The 3cDNMP task measures the ability to maintain spatial information across increasing delays. In the present study, a select group of old dogs was used that performed at an equivalent level to the young dogs on the 3cDNMP. On the spatial list tasks, which presumably engage both maintenance and executive functions (i.e., on-line monitoring processes) in working memory, these same old dogs were impaired relative to the young dogs. These performance differences between the different measures of visuospatial working memory suggest that the age-related impairment in spatial list learning may reflect deficits in executive functions engaged by complex working memory tasks, rather than maintenance processes per se. Selective aging of executive functions in the beagle dog was also observed in a recent size discrimination and reversal study conducted by our laboratory (Tapp et al. 2003).
Two types of errors were possible in the SLL tasks; errors could be made to the first presented object (primacy error) or to the second presented object (recency error). In both versions of the task, we found two consistent findings that parallel studies of list learning in other species. First, like humans, the pattern of primacy and recency effects did not change with age in the dog. In a study of list learning in aged adults, Korsnes and Magnussen (1996) reported that despite lower recall accuracy in old adults, the ratio of primacy-recency errors did not differ from young adults. Although our younger dogs performed at higher levels, the ratio of primacy-recency errors between young and old dogs did not differ. Second, memory for end items in the spatial list (recency effects) was better than memory for early list items (primacy effects) in both young and old dogs as indicated by greater primacy versus recency errors. Prominent recency effects also occur in studies of list learning in humans (Potter and Levy 1969; Weaver 1974; Hines 1975; Weaver and Stanny 1978; Roberts and Kraemer 1981; Wright et al. 1985; Wright 1999) and animals (Thompson and Herman 1977; Roberts and Smythe 1979; Buchanan et al. 1981; Roberts and Kraemer 1981; DiMattia and Kesner 1984; Santiago and Wright 1984; Wright et al. 1984, 1985; Bolhuis and van Kampen 1988; Deacon and Rawlins 1995; Matzke and Castro 1998; Wright 1999). The typical serial position function produced from list learning tasks emerges at delays of 10–20 sec. Beyond 30 sec, however, primacy effects (i.e., recency errors) are more robust (Roberts and Kraemer 1981; Santiago and Wright 1984; Wright et al. 1984, 1985; Bolhuis and van Kampen 1988; Deacon and Rawlins 1995; Wright 1998, 1999). We found, however, that varying the delay from 5–50 sec in the SLL tasks had no impact on the shape of the primacy-recency distribution; recency effects prevailed at all delays in both versions of the task.
Two important differences between the SLL protocols and standard list learning tasks may explain the prominent recency effects (i.e., primacy errors) obtained in the present study. First, the standard procedure for most list learning tasks involves presenting items serially, each separated by a very short interstimulus interval (e.g., 2–3 sec). Varying the delay interval between the list presentation and retest increases the memory demands in this standard procedure. In contrast, our tasks involved manipulating the length of the interstimulus interval between each spatial list item. Second, the number of stimulus items included on a list learning task impacts the ratio of primacy-recency errors. When short lists containing fewer than four items are used, proactive interference builds up quickly, reaching asymptotic levels within a few trials (Keppel and Underwood 1962; Sands and Wright 1980a), resulting in noticeably absent primacy effects (Roberts and Smythe 1979; Sands and Wright 1980a,b; Buchanan et al. 1981; Roberts and Kraemer 1981; Wright and Rivera 1997). On the SLL tasks, there were only three spatial positions tested per trial. Although the order of stimulus presentation varied from trial to trial, the same 3 positions were repeated on each of the 12 trials in a single session. When we analyzed the pattern of errors in each session, the total number of errors increased over the blocks of trials on the original SLL task. We have not previously reported this pattern of increasing within session errors from work in our laboratory. A likely suggestion is that memory for spatial items early in a session interfered with memory for spatial items that occurred later in each session (i.e., proactive interference). These proactive interference effects potentially confound age-related working memory explanations of impaired performance in the aged dog group, as aged populations are particularly sensitive to the effects of proactive interference (Hasher and Zacks 1988; Hartman and Hasher 1991; Hasher et al. 1991; McDowd and Filion 1992; Moscovitch and Winocur 1992, 1995; Connelly and Hasher 1993; May et al. 1999; Park and Hedden 2001). Thus, the inability of the old dogs to perform beyond short delays in the first study might reflect an increased susceptibility to proactive interference effects inherent in the original design of the SLL task. When we examined within session errors in the modified SLL, however, we found no evidence of proactive interference effects, but still found marked impairment in the old animals compared with the young.
Gender effects are commonly reported with performance on visuospatial tasks. Lacreuse et al. (1999) reported that young female monkeys perform worse than young males on a delayed recognition span task. The small number of females used in the present study, however, prohibits a meaningful examination of gender effects. The two females performed better than the males in the aged group, but the rigorous selection criteria may have selected for elite females. The young female dog performed worse than most of the young males, but conclusions drawn from a single dog are limited. Chan et al. (2002) reported that gender did not have a significant effect on the performance of the 3c-DNMP task in beagle dogs.
Our findings suggest that in the beagle dog, the working memory processes of maintenance and on-line monitoring are differentially affected by age. These dissociative effects may be linked to differential rates of aging in neuroanatomical regions that control maintenance and executive functions such as on-line monitoring. On-line monitoring and maintenance of information in working memory appear to depend on the dorsolateral and ventrolateral prefrontal cortex, respectively (Petrides et al. 1993; Owen et al. 1996, 1998, 1999; Cabeza et al. 1997; D'Esposito et al. 1999; Postle et al. 1999, 2000a,b,c; Rympa et al. 1999; Smith and Jonides 1999; Postle and D'Esposito 2000; Ross and Segalowitz 2000; Stern et al. 2000; Braver et al. 2001). The prefrontal cortex is particularly sensitive to the effects of aging (Kuhl et al. 1982; Duara et al. 1984; Coffey et al. 1992; Raz et al. 1993, 1997; Cowell et al. 1994; DeCarli et al. 1994). We have also obtained evidence that this cortical region is sensitive to aging in the dog (Head et al. 1998). Using magnetic resonance imaging techniques to quantify cortical volume, we recently found significant atrophy in the frontal lobes of the aged beagle dog (Tapp et al. 2002). Further work should establish whether this age-related pathology can be linked selectively to the proreal or subproreal cortical areas in the dog, which correspond to the primate dorsolateral and ventrolateral prefrontal cortices.
Increasing the amount and duration of information in working memory resulted in strong age-related decrements by use of two versions of a spatial-list task in the present study. Although memory in young and old dogs was greatest for end-list items, this pattern of responding likely reflects the length of the list used in the present study rather than a cognitive limitation in the beagle dog. Future studies of complex working memory for lists in the beagle dog would benefit from tests that use longer spatial lists, as well as tasks that examine list learning in nonspatial domains.
MATERIALS AND METHODS
Experiment I
Subjects
A total of 13 beagle dogs (Canis familiaris) were used. Seven dogs (four males, three females) were old dogs (aged 10.13–14.22 yr; M = 12.77, SD = 1.51) and six (five males, one female) were young dogs (aged 3.40–6.51 yr; M = 4.51, SD = 1.09). Subjects were housed singly in vivarium cages at the University of Toronto. Fresh water was available ad libitum. Each subject received ∼300 grams of food (Purina Dog Chow) following cognitive testing procedures. Temperatures and humidity in the housing facility were maintained at 21–24°C and 42%, respectively. Animals were inspected daily by trained veterinary and behavioral technicians. Clinical evaluations that included neurological examinations were conducted annually to assess somatosensory and motor functions. All dogs were in good health at the time of the study and showed no indications of sensory dysfunction that could have confounded the behavioral testing. All procedures were conducted in accordance with Canadian Council on Animal Care guidelines. All dogs had previous experience with several cognitive tasks, including the 2c and 3cDNMP tasks prior to this study.
Apparatus
Testing was conducted in a 0.609-m × 1.15-m × 1.08-m wooden canine adaptation of the Wisconsin General Test Apparatus as described previously (Milgram et al. 1994). The testing chamber was equipped with a sliding Plexiglas food tray containing three food wells, two lateral and one medial. The front of the box consisted of adjustable vertical stainless steel bars. The experimenter was separated visually from the dog by a screen with a one-way mirror and a hinged door on the bottom. Cognitive testing was conducted in darkness except for a light fixture with a 60-watt bulb attached to the front of the box. The hinged door was opened for the presentation and removal of the food tray. Approximately 1 cm3 of wet dog food (Hill's Prescription Diet p/d; Hill's Pet Nutrition Inc.) was used as the food reward.
Data was acquired using a dedicated computer program. The program controlled timing, randomization procedures, indicated the location of the reward, and was used to store and backup all data files.
Procedures
3cDNMP Pretraining
The 3cDNMP task was used to prescreen dogs for inclusion on the SLL task. Only dogs that successfully completed the 3cDNMP performed the SLL task. Procedures for the 3cDNMP task are described elsewhere (Chan et al. 2002). Briefly, each trial began with the presentation of a single object (in this case a red coffee jar lid) located on one of three food wells on the sliding tray. By displacing the object, the dog was able to obtain the food reward in the well beneath. The tray was withdrawn after the dog responded to the object, and after a brief delay, the tray was presented again with two red lids, one occupying the original location, and a second identical red lid occupying a second food well. Only responses to the object in the novel location were rewarded. Each dog completed a total of 12 trials per session. Pretraining measures were continued until a two-stage criterion was met. First, to reach criterion, a score of 11/12 or 12/12 on a single test session, 10/12 on two successive test sessions, or 10/12, 9/12, and 10/12 on three successive test sessions was required. To complete criterion, three additional test sessions of 70% or better were required. Any dog that failed to reach criterion within 40 d of training on the 3cDNMP task was removed from the study. Each dog was tested on delays of 5, 10, 20, and 30 sec during the pretraining procedures.
SLL Task
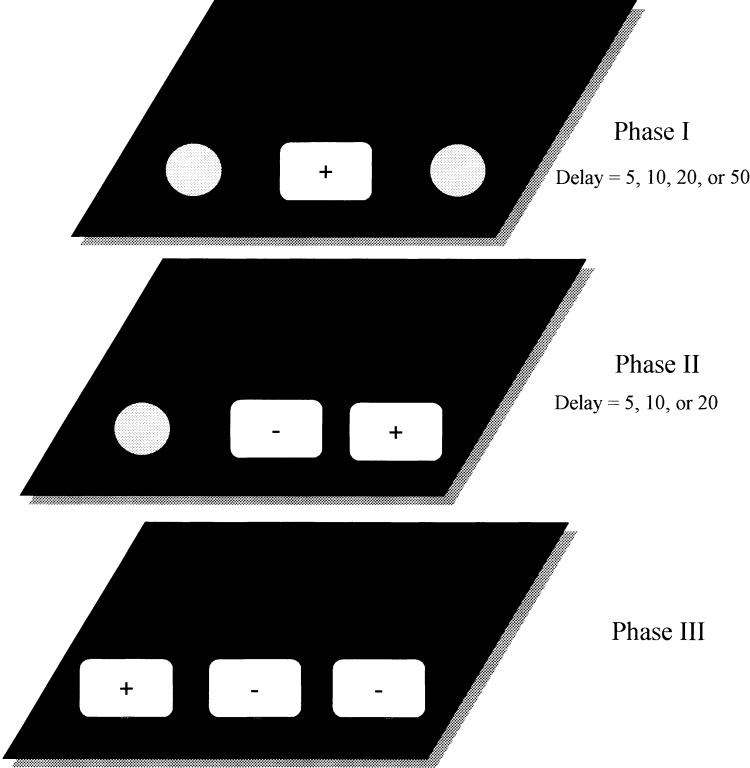
Testing on the SLL task commenced the first day following completion of 3cDNMP pretraining. All three food wells were used for the SLL task. Each dog received 1 session daily, 7 d per week, each consisting of 12 trials with 3 separate phases (Fig. 8). Phase one of each trial involved placing a single red lid over a food reward hidden in one of the three locations on the sliding tray. The hinged door was then raised, and the tray was pushed 1/3 of the way toward the dog and held there for 3 sec to allow the dog to inspect the tray. At the end of the inspection interval, the tray was pushed to the front of the box to allow the dog to respond. After a response was made, the tray was withdrawn for a fixed interval, after which phase two began. During phase two, the first lid was returned to the original position and a second lid was placed over one of the two remaining food wells. Only responses to the lid at the previously unrewarded location were rewarded. Following a response by the dog, the tray was removed for a second delay interval. On phase three of each SLL trial, the final well on the tray was baited and covered with a third identical red lid. The dog was then presented with the tray in which all three food wells were covered with identical red lids. To obtain the reward, the dog had to respond to the previously unrewarded (i.e., third) location. After the dog responded, the tray was removed for a 60-sec inter-trial interval before phase one of the next trial began. A single correction was permitted the first time an incorrect response was made on phase two and three within a session. All subsequent incorrect responses within a session were unrewarded. A small amount of food was smeared under each incorrect object on each trial to prevent the animal from using food odors to determine the location of the reward.
Figure 8.
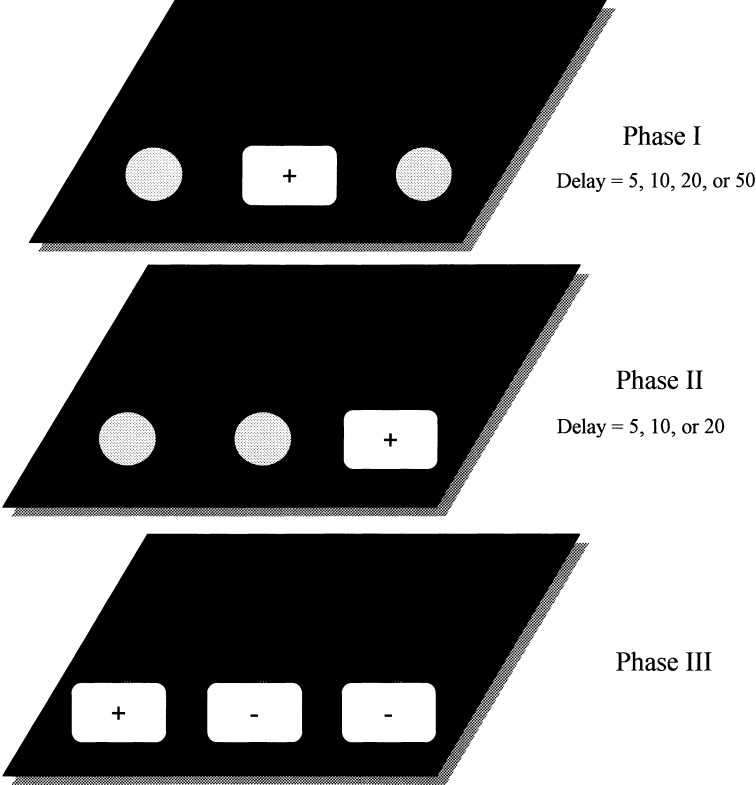
An example of a single trial on the original Spatial List Learning (SLL) task. Each trial consists of a series of three phases. In phase one, a single red coffee jar lid occupies one of the three food wells. In phase two, a second identical red lid is placed over one of the two remaining food wells. In the third and final phase of each trial, the last remaining food well is covered with a third identical red lid. Delays for each phase consist of 5–5, 10–10, 20–20, and 50–20 sec. Correct (+) and incorrect (−) positions on each phase are indicated.
Each dog received 12 trials per day for a maximum of 50 d. Testing on the SLL task began with 5-sec delay intervals between phases one and two and two and three of each trial. If criterion was met before 50 d of testing were completed, on subsequent testing, the delays were increased progressively to 10–10, 20–20, and 50–20 sec. Testing of a dog ended if the animal failed to reach criterion, or after the dog achieved criterion at the 50–20-sec delay.
Data Analysis
To examine acquisition, the total number of errors and trials to criterion were calculated separately for each delay interval. If an animal failed to reach criterion within the 50 d of scheduled testing, total errors and trials during the 50 d were used. For each dog, we calculated a maximal memory score on the basis of the longest delay interval that the dog was able to complete successfully within the allotted testing schedule. We also recorded the types of errors made by assessing both a primacy and recency score. Errors made to the first position in the spatial list during the third phase of each trial were recorded as primacy errors (i.e., recency effect); errors made to the second position were recorded as recency errors (i.e., primacy effects). To assess possible interference effects, we also distinguished total errors for trials within a session. Blocks of three trials were created so that trials 1–3, 4–6, 7–9, and 10–12 were grouped. Unless otherwise indicated, the Mann-Whitney U test for nonparametric data was used. Statistical analyses were performed using SPSS version 10.0.
Experiment II
Subjects
Of the original 12 beagle dogs used for the SLL task, 2 dogs (1 young male and 1 aged female) were unavailable for testing on the mSLL. Therefore, 10 of the initial 12 dogs that had completed the SLL task were included on the mSLL task. These 10 dogs consisted of 5 aged dogs (4 males, 1 female; M = 13.46, SD = 0.87) and 5 young dogs (4 males, 1 female; M = 4.63, SD = 1.21). Housing and feeding conditions were the same as those described for subjects in Experiment I.
Modified Spatial-List Learning Task
The apparatus and objects used were the same as those described for Experiment 1. Subjects received 12 daily trials composed of 3 phases (Fig. 9). On phase one, a single red lid was randomly placed over one of the three food wells. After making a response, the tray was withdrawn for a fixed interval. During the delay, a second location was baited and covered with a second red lid. The position from phase one, however, remained empty. Thus, after the delay, only a single red lid in one of the two remaining positions was presented. After a second delay, the third remaining position was baited and covered with a red lid. The first two positions were also covered with a red lid, and the tray was presented to the dog. Only responses to the third novel position were rewarded. A single correction was permitted per session the first time an incorrect response was made during the third phase of a trial. Randomization, criterion measures, and data collection procedures were identical to those described for the original SLL task.
Figure 9.
An example of a single trial on the modified SLL (mSLL) task. Each trial is composed of a series of three phases. In phase one, a single red coffee jar lid occupies one of the three food wells. In phase two, a second identical red lid is placed over one of the two remaining food wells. During this phase, the first position is not covered by a red lid. In the third and final phase of each trial, the last remaining food well is covered with a third identical red lid, and the first two positions are covered with red lids, but remain unbaited. Delays for each phase consist of 5–5, 10–10, 20–20, and 50–20 sec. Correct (+) and incorrect (−) positions on each phase are indicated.
Data Analysis
The same analyses were performed as for the original SLL task.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL milgram@psych.utoronto.ca; FAX (416) 287-7642.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.56503.
REFERENCES
- Adams B, Chan A, Callahan H, Milgram NW. The canine as a model of human cognitive aging: Recent developments. Prog Neuropsychopharmacol Biol Psychiatry. 2000a;24:675–692. doi: 10.1016/s0278-5846(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Adams B, Chan A, Callahan H, Siwak C, Tapp D, Ikeda-Douglas C, Atkinson P, Head E, Cotman CW, Milgram NW. Use of a delayed non-matching to position task to model age-dependent cognitive decline in the dog. Behav Brain Res. 2000b;108:47–56. doi: 10.1016/s0166-4328(99)00132-1. [DOI] [PubMed] [Google Scholar]
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychol Aging. 1990;5:412–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford, UK: Clarendon Press; 1986. [Google Scholar]
- ————— . Levels of working memory. In: Naveh-Benjamin M, Moscovitch M, Roediger III HL, editors. Perspectives on human and cognitive aging: Essays in honour of Fergus Craik. New York, NY: Psychology Press; 2001. pp. 111–123. [Google Scholar]
- Beason, L., Moss, M.B., and Rosene, D.L. 1990. Effects of entorhinal, parahippocampal, or basal forebrain lesions on recognition memory in the monkey. Soc. Neurosci. Abstr. 16.
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Becker JT, Huff FJ, Nebes RD, Holland A, Boller F. Neuropsychological function in Alzheimer's disease: Pattern of impairment and rates of progression. Arch Neurol. 1988;45:263–268. doi: 10.1001/archneur.1988.00520270037018. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, van Kampen HS. Serial position curves in spatial memory of rats: Primacy and recency effects. Q J Exp Psychol B. 1988;40:135–149. [PubMed] [Google Scholar]
- Botwinick J. Aging and behavior. New York, NY: Springer Publishing; 1984. pp. 336–358. [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Meizin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, et al. Direct comparison of prefrontal cortex regions engaged by working memory and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Brouwers P, Cox C, Martin A, Chase T, Fedio P. Differential perceptual-spatial impairment in Huntington's and Alzheimer's dementias. Arch Neurol. 1984;41:1073–1076. doi: 10.1001/archneur.1984.04050210071017. [DOI] [PubMed] [Google Scholar]
- Buchanan JP, Gill TV, Braggio JT. Serial position and clustering effects in a chimpanzee's free recall. Mem Cognit. 1981;9:651–660. doi: 10.3758/bf03202360. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, Tulving E. Brain regions involved in remembering what and when: A PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Callahan H, Ikeda-Douglas C, Head E, Cotman CW, Milgram NW. Development of a protocol for studying object recognition memory in the dog. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:693–707. doi: 10.1016/s0278-5846(00)00102-0. [DOI] [PubMed] [Google Scholar]
- Castro CA. Primacy and recency effects in rhesus monkeys (Macaca mulatta) using a serial probe recognition task I: Effects of diazepam. Psychopharmacology. 1995;119:421–427. doi: 10.1007/BF02245858. [DOI] [PubMed] [Google Scholar]
- ————— Primacy and recency effects in rhesus monkeys (Macaca mulatta) using a serial probe recognition task II: Effects of atropine sulphate. Behav Neurosci. 1997;111:676–682. doi: 10.1037//0735-7044.111.4.676. [DOI] [PubMed] [Google Scholar]
- Castro CA, Larsen T. Primacy and recency effects in nonhuman primates. J Exp Psychol Anim Behav Process. 1992;18:335–340. doi: 10.1037//0097-7403.18.4.335. [DOI] [PubMed] [Google Scholar]
- Chan ADF, Nippak PMD, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW. Visuspatial impairments in aged canines (Canis familiaris): The role of Cognitive-behavioral flexibility. Behav Neurosci. 2002;116:443–454. [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Parashos IA, Soady SAR, Sullivan RJ, Patterson LJ, Figiel GS, Webb MC, Spritzer CE, Djang WT. Quantitative cerebral anatomy of the aging brain: A cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L. Aging and the inhibition of spatial location. J Exp Psychol Hum Percept Perform. 1993;19:1238–1250. doi: 10.1037//0096-1523.19.6.1238. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging in the human frontal and temporal lobes. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 51–110. [Google Scholar]
- Craik FIM, Morris RG, Gick ML. Adult age differences in working memory. In: Vallar G, Shallice T, editors. Neuropsychological impairments of short-term memory. Cambridge, UK: Cambridge University Press; 1990. pp. 247–267. [Google Scholar]
- Crowder RG. Principles of learning and memory. Hillsdale, NJ: Erlbaum; 1976. [Google Scholar]
- Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996;17:259–268. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP. Serial position effects and duration of memory for nonspatial stimuli in rats. J Exp Psychol Anim Behav Process. 1995;21:285–292. doi: 10.1037//0097-7403.21.4.285. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DGM, Gillette JA, Haxby JV, Teichberg D, Schapiro MB, Horwitz B. Lack of age-related differences in temporal lobe volume of very healthy adults. Am J Neuroradiol. 1994;15:689–696. [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- DiMattia BV, Kesner RP. Serial position curves in rats: Automatic versus effortful information processing. J Exp Psychol Anim Behav Process. 1984;10:557–563. [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Duara R, Grady C, Haxby J, Ingvar D, Sokoloff L, Margolin R, Manning RG, Cutler NR, Rapoport SI. Human brain glucose utilization and cognitive function in relation to age. Ann Neurol. 1984;16:702–713. doi: 10.1002/ana.410160613. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus HE. Grundzuge der Psychologie (Basic Psychology). Leipzig, Germany: Von Veit; 1902. [Google Scholar]
- ————— . Memory: A contribution to experimental psychology. New York, NY: Dover Publications; 1964. [Google Scholar]
- Eslinger PJ, Benton AL. Visuoperceptual performance in aging and dementia: Clinical and theoretical observations. J Clin Neuropsychol. 1983;5:213–220. doi: 10.1080/01688638308401170. [DOI] [PubMed] [Google Scholar]
- Estrada J, Ikeda-Douglas C, Milgram NW, Zicker SC. The effects of diet and age on the performance of the landmark discrimination learning task. Soc Neurosci Abstr. 2001;27:101.9. [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: Predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- Freedman M, Oscar-Berman M. Spatial and visual deficits in Alzheimer's and Parkinson's disease. Brain Cogn. 1989;11:114–126. doi: 10.1016/0278-2626(89)90009-2. [DOI] [PubMed] [Google Scholar]
- Frieske DA, Park DC. Effects of organization and working memory on age differences in memory for scene information. Exp Aging Res. 1993;19:321–332. doi: 10.1080/03610739308253941. [DOI] [PubMed] [Google Scholar]
- Gick ML, Craik FIM, Morris RG. Task complexity and age differences in working memory. Mem Cognit. 1988;16:353–361. doi: 10.3758/bf03197046. [DOI] [PubMed] [Google Scholar]
- Hartman M, Hasher L. Aging and suppression: Memory for previously relevant information. Psychol Aging. 1991;6:587–594. doi: 10.1037//0882-7974.6.4.587. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Mohs RC. Memory changes with aging and dementia. In: Hof PR, Mobbs CV, editors. Functional neurobiology of aging. New York, NY: Academic Press; 2001. pp. 53–63. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower G, editor. The psychology of learning and motivation. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rympa B. Age and inhibition. J Exp Psychol Learn, Mem Cognit. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Head E, Mehta R, Hartley J, Kameka M, Cummings BJ, Cotman C, Reid C, Milgram NW. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and β-Amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Head E, Milgram NW, Cotman CW. Neurobiological models of aging in the dog and other vertebrate species. In: Hof PR, Mobbs CV, editors. Functional neurobiology of aging. New York, NY: Academic Press; 2001. pp. 457–468. [Google Scholar]
- Herndon JG, Killiany RJ, Rosene DL, Moss MB. The recognition span memory capacity in of intermediate aged (19–24 years old) rhesus monkeys. Soc Neurosci Abstr. 1993;19:600. [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hines D. Immediate and delayed recognition of sequentially presented random shapes. J Exp Psychol Hum Learn Mem Cog. 1975;1:634–639. [PubMed] [Google Scholar]
- Huff FJ, Becker JT, Belle SH, Nebes RD, Holland AL, Boller F. Cognitive deficits and clinical diagnosis of Alzheimer's disease. Neurology. 1987;37:1119–1124. doi: 10.1212/wnl.37.7.1119. [DOI] [PubMed] [Google Scholar]
- Keppel G, Underwood BJ. Proactive inhibition in short-term retention of single items. J Verbal Learn Verbal Behav. 1962;1:152–161. [Google Scholar]
- Kesner RP, Holbrook T. Dissociation of item and order spatial memory in rats following medial prefrontal cortex lesions. Neuropsychologia. 1987;25:653–664. doi: 10.1016/0028-3932(87)90056-x. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Novak JM. Serial position curve in rats: Role of the dorsal hippocampus. Science. 1982;218:173–175. doi: 10.1126/science.7123228. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Measom MO, Forsman SL, Holbrook TH. Serial-position curves in rats: Order memory for episodic spatial events. Anim Learn Behav. 1984;12:378–382. [Google Scholar]
- Korsnes MS, Magnussen S. Age comparisons of serial position effects in short-term memory. Acta Psychol. 1996;94:133–143. doi: 10.1016/0001-6918(95)00056-9. [DOI] [PubMed] [Google Scholar]
- Kuhl D, Metter E, Riege W, Phelps M. Effects of human aging on patterns of local cerebral glucose utilization determined by the fluorodeoxyglucose method. J Cereb Blood Flow Metab. 1982;2:163–171. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Henrdon JG, Killiany R, Rosene DL, Moss MB. Spatial cognition in rhesus monkeys: Male superiority declines with age. Horm Behav. 1999;36:70–76. doi: 10.1006/hbeh.1999.1532. [DOI] [PubMed] [Google Scholar]
- Martin A. Representation of semantic and spatial knowledge in Alzheimer's patients: Implications for models of preserved learning in amnesia. J Clin Exp Neuropsychol. 1987;9:191–224. doi: 10.1080/01688638708405361. [DOI] [PubMed] [Google Scholar]
- Matzke SM, Castro CA. Primacy and recency effects in rhesus monkeys (Macaca mulatta) using a serial probe recognition task III. A Developmental analysis. Dev Psychobiol. 1998;32:215–224. doi: 10.1002/(sici)1098-2302(199804)32:3<215::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Kane MJ. The role of interference in memory span. Mem Cognit. 1999;27:759–767. doi: 10.3758/bf03198529. [DOI] [PubMed] [Google Scholar]
- Mazaux JM, Dartigues JF, Letenneur L, Darriet D, Wiart L, Gagnon M, Commenges D, Boller F. Visuo-spatial attention and psychomotor performance in elderly community residents: Effects of age, gender, and education. J Clin Exp Neuropsychol. 1995;17:71–81. doi: 10.1080/13803399508406583. [DOI] [PubMed] [Google Scholar]
- McDowd JM, Filion DL. Aging, selective attention, and inhibitory processes: A psychophysiological approach. Psychol Aging. 1992;7:65–71. doi: 10.1037//0882-7974.7.1.65. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Mendez MA, Martin RN, Smyth KA, Whitehouse PJ. Complex visual disturbances in Alzheimer's disease. Neurology. 1990;40:439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog: Acquisition of non-spatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Adams B, Callahan H, Head E, Mackay B, Thirlwell C, Cotman CW. Landmark discrimination learning in the dog. Learn Mem. 1999;6:54–61. [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Estrada J, Ikeda-Douglas C, Castillo J, Head E, Cotman CW, Murphey H, Holowachuk D, Muggenburg BA, Zicker SC. Landmark discrimination learning in aged dogs is improved by treatment with an antioxidant enriched diet. Soc Neurosci Abstr. 2000;26:193.9. [Google Scholar]
- Milgram NW, Head E, Muggenburg B, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: Effects of age, antioxidant fortified food and cognitive strategy. Neurosci Biobehav Rev. 2002;26:679–695. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Morrell RW, Park DC. The effects of age, illustrations, and task variables on the performance of procedural assembly tasks. Psychol Aging. 1993;8:389–399. doi: 10.1037//0882-7974.8.3.389. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Erlbaum Associates; 1992. pp. 315–372. [Google Scholar]
- ————— . Frontal lobes, memory, and aging. In: Grafman J, Holyoak K, Bohler F, editors. Structure and functions of the human prefrontal cortex. New York, NY: New York Academy of Sciences; 1995. pp. 119–150. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon JG. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18:13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Cognitive dysfunction in Alzheimer's disease. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 373–446. [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral cortex: A positron emission tomography study. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey L, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processes within the human lateral cortex. Proc Natl Acad Sci. 1998;95:7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark CJ, Downey SPMJ, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, et al. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Park DC, Hedden T. Working memory and aging. In: Naveh-Benjamin M, Moscovitch M, Roediger III HL, editors. Perspectives on human memory and cognitive aging: Essays in honor of Fergus Craik. New York, NY: Psychology Press; 2001. pp. 148–160. [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl W. Dissociation of spatial discrimination deficits following frontal and parietal lesions in monkeys. J Comp Physiol Psychol. 1973;82:227–239. doi: 10.1037/h0033922. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M. Evaluating models of the topographical organization of working memory function in frontal cortex with event-related fMRI. Psychobiology. 2000;28:132–145. [Google Scholar]
- Postle BR, Berger JS, D'Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci. 1999;96:12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D'Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behaviour. J Cogn Neurosci. 2000a;12:2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corken S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. Neuroimage. 2000b;11:409–423. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Res Protoc. 2000c;5:57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Potter MC, Levy EI. Recognition memory for a rapid sequence of pictures. J Exp Psychol. 1969;81:10–15. doi: 10.1037/h0027470. [DOI] [PubMed] [Google Scholar]
- Raffel G. Two determinants of the effect of primacy. Am J Psychol. 1936;48:654–657. [Google Scholar]
- Raz N, Torres IJ, Spencer WD, Acker JD. Pathoclysis in aging human cerebral cortex: Evidence from in vivo MRI morphometry. Psychobiology. 1993;21:151–160. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal grey matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Kraemer PJ. Recognition memory for lists of visual stimuli in monkeys and humans. Anim LearnBehav. 1981;9:587–594. [Google Scholar]
- Roberts WA, Smythe WE. Memory for lists of spatial events in the rat. Learn Motiv. 1979;10:313–316. [Google Scholar]
- Ross P, Segalowitz SJ. An EEG coherence test of the frontal dorsal versus ventral hypothesis in n-back working memory. Brain and Cogn. 2000;43:130–134. [PubMed] [Google Scholar]
- Rympa B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli DE. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Working memory mediation of adult age differences in integrative reasoning. Mem Cognit. 1992;20:413–423. doi: 10.3758/bf03210925. [DOI] [PubMed] [Google Scholar]
- ————— Influence of working memory on adult age differences in matrix reasoning. Br J Psychol. 1993;84:171–199. doi: 10.1111/j.2044-8295.1993.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- Salthouse TA, Mitchell DR, Skovronek E, Babcock RL. Effects of adult age and working memory on reasoning and spatial abilities. J Exp Psychol Learn Mem Cogn. 1989;15:507–516. doi: 10.1037//0278-7393.15.3.507. [DOI] [PubMed] [Google Scholar]
- Sands SF, Wright AA. Serial probe recognition performance by a rhesus monkey and a human with 10 and 20-item lists. J Exp Psychol Anim Behav Process. 1980a;6:386–396. [PubMed] [Google Scholar]
- ————— Primate memory: Retention of serial list items by a rhesus monkey. Science. 1980b;209:938–940. doi: 10.1126/science.6773143. [DOI] [PubMed] [Google Scholar]
- Santiago HC, Wright AA. Pigeon memory: Same/different concept learning, serial probe acquisition, and probe delay effects on the serial-position function. J Exp Psychol Anim Behav Process. 1984;10:498–512. [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: Evidence from functional magnetic resonance imaging. Neuroimage. 2000;11:392–399. doi: 10.1006/nimg.2000.0569. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Chiou G, Black SE, McCune S, Head E, Cotman CW, Milgram NW, Su Y-M. Effects of age on frontal and hemispheric brain symmetry in the beagle dog. Soc Neurosci Abstr. 2002;28:374.9. [Google Scholar]
- Tapp, P.D., Siwak, C.T., Estrada, J., and Milgram, N.W. 2003. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. 10: 64–73. [DOI] [PMC free article] [PubMed]
- Terrace HS, Chen S, Newman AB. Serial learning with a wild card by pigeons (Columbia livia): Effect of list length. J Comp Psychol. 1995;109:162–172. doi: 10.1037/0735-7036.109.2.162. [DOI] [PubMed] [Google Scholar]
- Thompson RKR, Herman LM. Memory for lists of sounds by the bottle-nosed dolphin: Convergence of memory processes with humans? Science. 1977;195:501–503. doi: 10.1126/science.835012. [DOI] [PubMed] [Google Scholar]
- Weaver GE. Effects of post-stimulus study time on recognition of pictures. J Exp Psychol. 1974;103:799–801. [Google Scholar]
- Weaver GE, Stanny CJ. Short-term retention of pictorial stimuli as assessed by a probe recognition technique. J Exp Psychol Hum Learn Mem. 1978;4:55–65. [Google Scholar]
- Welch GB, Burnett CT. Is primacy a factor in association-formation? Am J Psychol. 1942;35:396–401. [Google Scholar]
- Wright AA. Auditory list memory in rhesus monkeys. Psychol Sci. 1998;9:91–98. [Google Scholar]
- ————— Visual list memory in capuchin monkeys (Cebus apella) J Comp Psychol. 1999;113:74–80. doi: 10.1037/0735-7036.113.1.74. [DOI] [PubMed] [Google Scholar]
- Wright AA, Rivera JJ. Memory for auditory lists by rhesus monkeys (Macaca mulatta) J Exp Psychol Anim Behav Process. 1997;23:441–449. doi: 10.1037//0097-7403.23.4.441. [DOI] [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF. Monkey memory: Same/different concept learning, serial probe acquisition, and probe delay effects. J Exp Psychol Anim Behav Process. 1984;10:513–529. [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory of serial lists by pigeons, monkeys, and people. Science. 1985;229:287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]