Abstract
The endogenous cannabinoid system has been shown recently to play a crucial role in the extinction of aversive memories. As the amygdala is presumably involved in this process, we investigated the effects of the cannabinoid receptor agonist WIN 55,212-2 (WIN-2) on synaptic transmission in the lateral amygdala (LA) of wild-type and cannabinoid receptor type 1 (CB1)-deficient mice. Extracellular field potential recordings and patch-clamp experiments were performed in an in vitro slice preparation. We found that WIN-2 reduces basal synaptic transmission and pharmacologically isolated AMPA receptor- and GABAA receptor-mediated postsynaptic currents in wild-type, but not in CB1-deficient mice. These results indicate that, in the LA, cannabinoids modulate both excitatory and inhibitory synaptic transmission via CB1. WIN-2-induced changes of paired-pulse ratio and of spontaneous and miniature postsynaptic currents suggest a presynaptic site of action. Inhibition of Gi/o proteins and blockade of voltage-dependent and G protein-gated inwardly rectifying K+ channels inhibited WIN-2 action on basal synaptic transmission. In contrast, modulation of the adenylyl cyclase-protein kinase A pathway, and blockade of presynaptic N- and P/Q- or of postsynaptic L- and R/T-type voltage-gated Ca2+ channels did not affect WIN-2 effects. Our results indicate that the mechanisms underlying cannabinoid action in the LA partly resemble those observed in the nucleus accumbens and differ from those described for the hippocampus.
Cannabinoids display a variety of central effects such as impairment of hippocampus-dependent learning and memory, modulation of emotional states and analgesia (Breivogel and Childers 1998; Pertwee 2001; Porter and Felder 2001). They inhibit stimulus-evoked synaptic transmission in several brain regions such as the hippocampus (Misner and Sullivan 1999), nucleus accumbens (Robbe et al. 2001) and prefrontal cortex (Auclair et al. 2000) predominantly via presynaptic mechanisms. Most behavioral effects of cannabinoids are mediated by the cannabinoid receptor type 1 (CB1; Ledent et al. 1999; Zimmer et al. 1999). Activation of the Gi/o protein-coupled CB1 inhibits the adenylyl cyclase-protein kinase A (AC-PKA) pathway (Howlett et al. 1986) and modulates Ca2+ and K+ conductances (Deadwyler et al. 1995; Mackie et al. 1995; McAllister et al. 1999). CB1 is widely distributed throughout the central nervous system. Among other brain regions, CB1 is highly expressed in the amygdala (Marsicano and Lutz 1999; Katona et al. 2001; McDonald and Mascagni 2001), which is an integral component of the limbic circuitry.
The amygdala plays a major role in the control of emotional behavior, including conditioned fear and anxiety (Davis et al. 1994; McKernan and Shinnick-Gallagher 1997; Rogan et al. 1997), and pain perception (Martin et al. 1999; Becerra et al. 2001; Manning et al. 2001; Gauriau and Bernard 2002; Paulson et al. 2002). In a recent investigation using auditory fear-conditioning tests, we found that the endogenous cannabinoid system is crucially involved in the extinction of aversive memories (Marsicano et al. 2002). Endocannabinoids are considered to be retrograde messengers released by neurons to modulate release of neurotransmitters (Kreitzer and Regehr 2001; Ohno-Shosaku et al. 2001; Wilson and Nicoll 2001). In the amygdala, immunohistochemical and electrophysiological findings showed that CB1 protein is mainly present in a subpopulation of GABAergic interneurons, and that CB1 activation modulates GABAergic synaptic transmission (Katona et al. 2001). However, in this brain region, CB1 mRNA is also detected in non-GABAergic cells (Marsicano and Lutz 1999), suggesting the possibility of CB1-mediated control of glutamatergic synaptic transmission. Hajos et al. (2001) showed that in the hippocampus of CB1-deficient mice, the synthetic cannabinoid agonist WIN-2 did not reduce GABAergic synaptic transmission anymore, whereas it was still able to affect glutamatergic synaptic transmission. The authors concluded that cannabinoid actions on glutamatergic synaptic transmission in the hippocampus are not mediated by CB1. In the amygdala, the effects and underlying mechanisms of CB1 activation on both isolated glutamatergic and GABAergic synaptic transmission have not yet been investigated. We used whole-cell patch-clamp recording from principal neurons and extracellular recording techniques to investigate cannabinoid actions on basal, as well as on isolated glutamatergic and GABAergic synaptic transmission in the LA of wild-type and CB1-deficient mice.
RESULTS
Effects of CB1 Activation on Basal Synaptic Transmission and Isolated Glutamatergic Synaptic Transmission
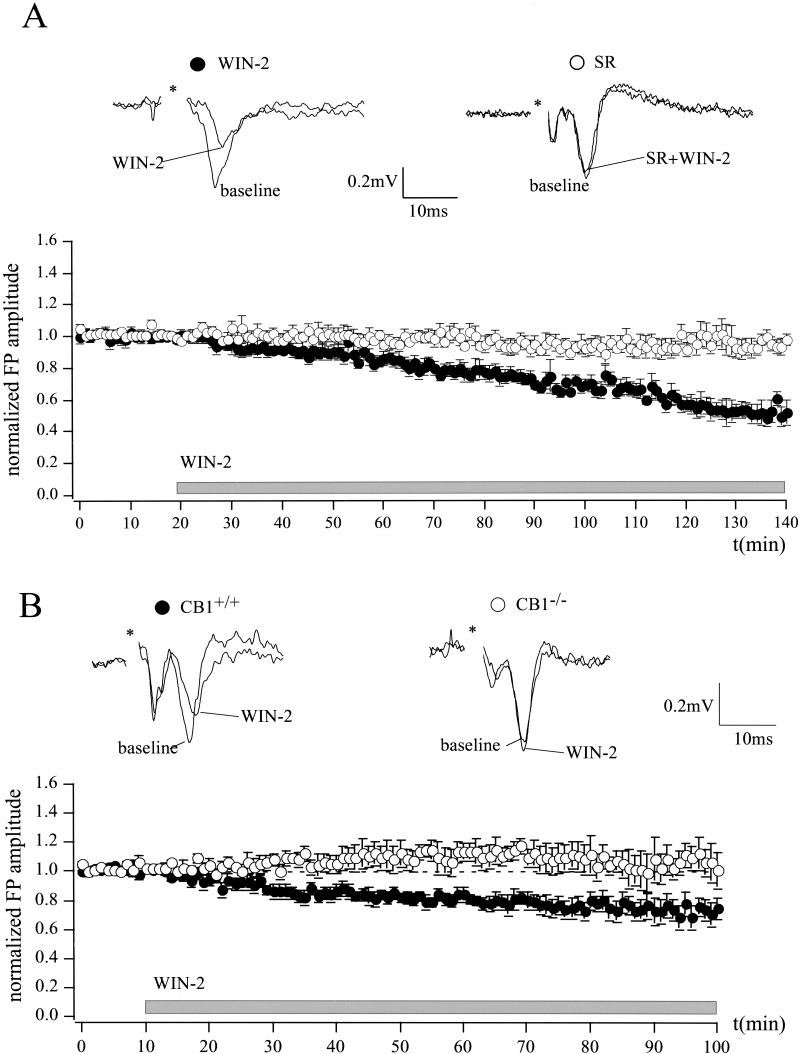
The effects of CB1 activation on the activity of a population of neurons in the LA were investigated by extracellular recording of field potentials (FPs). The CB1 agonist WIN-2 (5 μM) significantly reduced FP amplitude to 52 ± 6% of baseline (n = 7; P < 0.05; Fig. 1A). The CB1 antagonist SR 141716A (SR; 5 μM) abolished this effect (baseline, 100%; WIN-2 + SR, 94 ± 5%; n = 7; P > 0.05). SR alone did not affect the FP amplitude (97 ± 2% of baseline; n = 4; P > 0.05; data not shown). Because it was shown that SR also reversed WIN-2-induced effects in the hippocampus of mice lacking CB1 (Hajos et al. 2001), we additionally compared WIN-2 actions in the LA of wild-type (CB1+/+) and CB1-deficient (CB1−/−) mice (Fig. 1B). WIN-2 significantly reduced FP amplitude in CB1+/+ mice to 69 ± 9% of baseline (n = 5; P < 0.05), but not in CB1−/− mice (102 ± 12% of baseline; n = 4; P > 0.05; effect of WIN-2 on CB1+/+ versus CB1−/−; P < 0.05). We also tested the effect of WIN-2 (5 μM) on evoked excitatory postsynaptic currents (eEPSCs) from principal neurons in the LA by whole-cell patch-clamp recording. Principal neurons were distinguished from interneurons according to their morphological and electrophysiological properties (Washburn and Moises 1992). WIN-2 reduced eEPSC amplitude to 71 ± 10% of baseline levels (n = 6; P < 0.05) and increased paired-pulse ratio (PPR), an index for an alteration of presynaptic transmitter release (Manabe et al. 1993; Asztely et al. 1996), from 1.08 ± 0.1 to 1.27 ± 0.13 (n = 6; P < 0.05; Fig. 2A).
Figure 1.
Cannabinoid-induced inhibition of synaptic transmission in the mouse LA. (A) The CB1 agonist WIN-2 (5 μM) reduces FP amplitude to 52 ± 6% of baseline (n = 7; P < 0.05), as measured by extracellular recordings. This effect is completely abolished in the presence of the CB1 antagonist SR (SR 5 μM + WIN-2 5 μM, 94 ± 5%; n = 7; P > 0.05), which itself does not have any effect on FP amplitude (97 ± 2% of baseline; n = 4; data not shown). (B) WIN-2 significantly reduces FP amplitude in CB1+/+ mice to 69 ± 9% of baseline (n = 5; P < 0.05), but not in CB1−/− mice (102 ± 12% of baseline; n = 4; P > 0.05; effect of WIN-2 on CB1−/− vs. CB1+/+; P < 0.05). Representative traces are shown. All data are normalized to the respective baseline values (last 10 min of baseline). Asterisks represent stimulation artifacts. Gray bar shows period of WIN-2 superfusion.
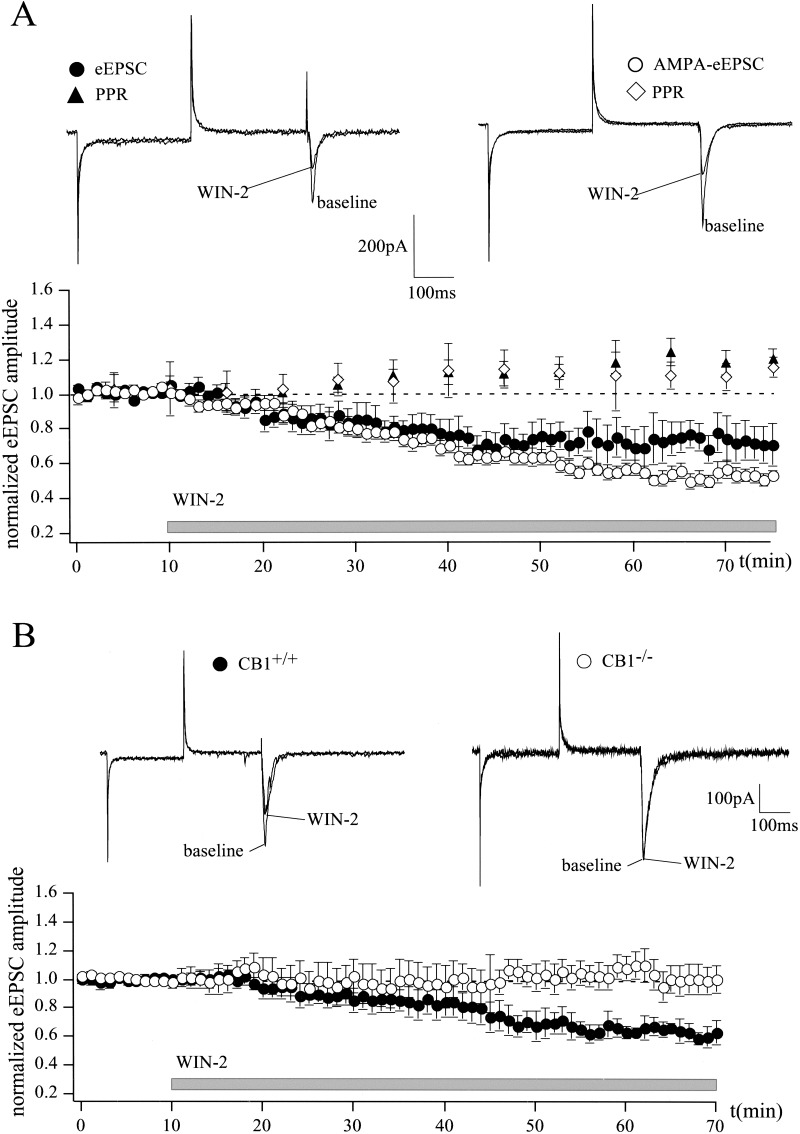
Figure 2.
Cannabinoids inhibit glutamatergic synaptic transmission in the LA. (A) Whole-cell patch-clamp recordings show that WIN-2 (5 μM) reduces eEPSC amplitude to 71 ± 10% (n = 6; P < 0.05) and AMPA-eEPSC amplitude to 52 ± 4% (n = 6; P < 0.05) of baseline. WIN-2 enhances PPRs of eEPSCs (from 1.08 ± 0.1 to 1.27 ± 0.13; n = 6; P < 0.05) and AMPA-eEPSCs (from 0.99 ± 0.03 to 1.18 ± 0.04; n = 6; P < 0.05). (B) WIN-2 (5 μM) has no effect on AMPA receptor-mediated synaptic transmission in CB1−/− mice (106 ± 14% of baseline; n = 5; P > 0.05), whereas it significantly reduces AMPA-eEPSC amplitude in the CB1+/+ mice to 59 ± 3% of baseline (n = 4; P < 0.05). Representative traces are shown. All data are normalized to the respective baseline values (last 10 min of baseline). Gray bar shows period of WIN-2 superfusion.
FPs and eEPSCs reflect the concerted action of both glutamatergic and GABAergic synaptic transmission. To investigate the effects of WIN-2 on the two functionally antagonistic systems, isolated AMPA receptor-mediated currents (AMPA-EPSCs) and GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) were recorded. The effect of WIN-2 (5 μM) on evoked AMPA-eEPSCs was studied in the presence of the GABAA receptor antagonist picrotoxin (50 μM), the NMDA receptor antagonist D-AP5 (50 μM) and the GABAB receptor antagonist CGP 35348 (200 μM). WIN-2 significantly reduced AMPA-eEPSC amplitude to 52 ± 4% of baseline (n = 6; P < 0.05) and increased PPR from 0.99 ± 0.03 to 1.18 ± 0.04 (n = 6; P < 0.05), suggesting that WIN-2 directly inhibits glutamatergic synaptic transmission in the LA (Fig. 2A). In contrast to Hajos et al. (2001), who showed that WIN-2 still affected glutamatergic synaptic transmission in the hippocampus of CB1-deficient mice, we found that WIN-2 (5 μM) had no effect on AMPA receptor-mediated synaptic transmission in the LA of CB1−/− mice (106 ± 14% of baseline; n = 5; P > 0.05). Under control conditions (CB1+/+), WIN-2 significantly reduced AMPA-eEPSC amplitude to 59 ± 3% of baseline level (n = 4; P < 0.05; Fig. 2B). These results indicate that, in contrast to the hippocampus, the observed WIN-2-induced decrease of glutamatergic synaptic transmission in the LA is CB1-mediated.
Effects of CB1 Activation on GABAergic Synaptic Transmission
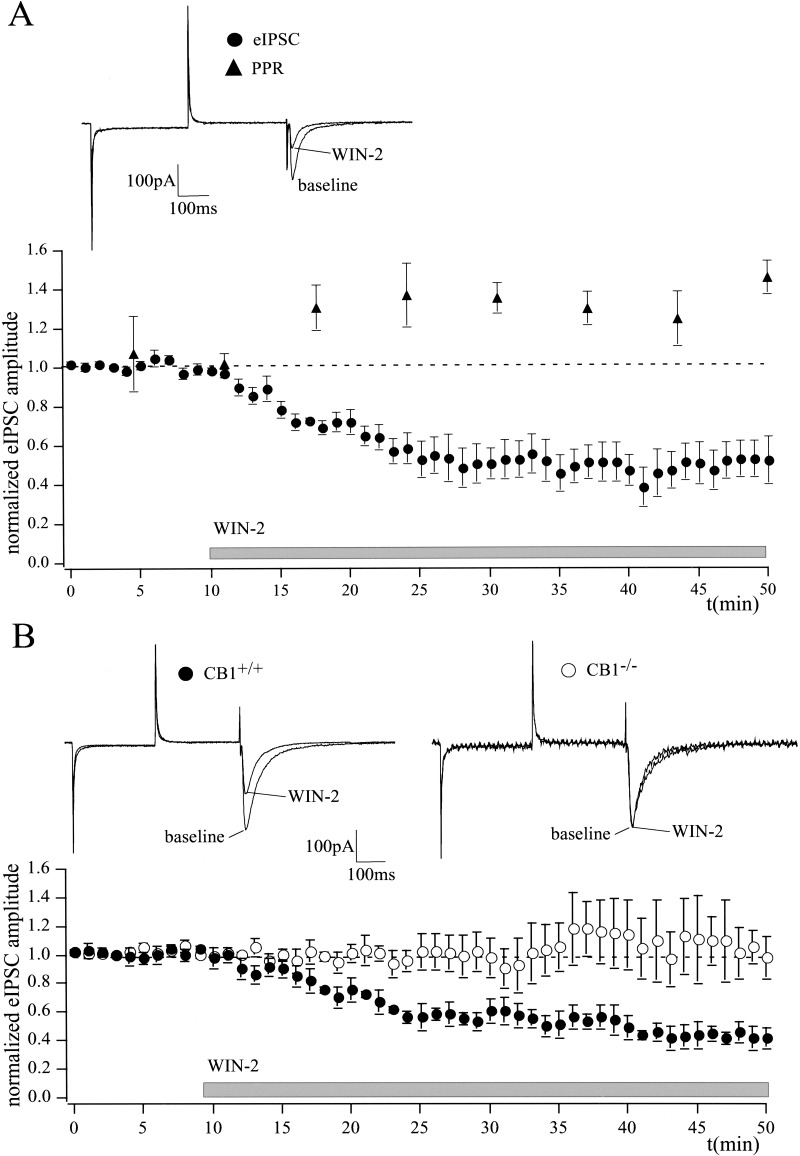
Cannabinoids inhibit GABAergic synaptic transmission in different brain regions (Hoffman and Lupica 2000; Katona et al. 2001; Manzoni and Bockaert 2001). Here, we investigated cannabinoid effects on isolated GABAergic synaptic transmission in the LA. Evoked GABAA receptor-mediated inward currents (eIPSCs) were measured in the LA at a holding potential of −70 mV with high intracellular Cl−. NMDA receptors, AMPA/kainate receptors, and GABAB receptors were antagonized by D-AP5 (50 μM), NBQX (5 μM), and CGP 35348 (200 μM), respectively. Bath application of WIN-2 (5 μM) significantly reduced eIPSC amplitude to 50 ± 10% of baseline (n = 8; P < 0.05) and enhanced PPR from 1.02 ± 0.01 to 1.5 ± 0.06 (n = 5; P < 0.05; Fig. 3A). The effects were reversed to 93 ± 5% of baseline (n = 6; P > 0.05; data not shown) by the CB1 antagonist SR (5 μM). Figure 3B shows that, in the LA, WIN-2 (5 μM) significantly reduced eIPSC amplitude in wild-type animals to 47 ± 6% of baseline (n = 4; P < 0.05), but not in CB1-deficient mice (105 ± 15% of baseline; n = 4; P > 0.05).
Figure 3.
Cannabinoids inhibit GABAergic synaptic transmission in the LA. (A) WIN-2 (5 μM) decreases eIPSC amplitude to 50 ± 10% (n = 8; P < 0.05) of baseline, as measured by whole-cell patch-clamp recording. The agonist also enhances PPR of eIPSCs (control, 1.02 ± 0.01; WIN-2, 1.5 ± 0.06; n = 5; P < 0.05), suggesting a presynaptic inhibition of GABAergic synaptic transmission. (B) WIN-2 (5 μM) significantly reduces eIPSC amplitude in the CB1+/+ mice to 47 ± 6% of baseline (n = 4; P < 0.05) without affecting GABAergic synaptic transmission in CB1−/− mice (105 ± 15% of baseline; n = 4; P > 0.05). Representative traces are shown. All data are normalized to the respective baseline values (last 10 min of baseline). Gray bar shows period of WIN-2 superfusion.
Mechanisms Underlying the Cannabinoid Effects on Synaptic Transmission
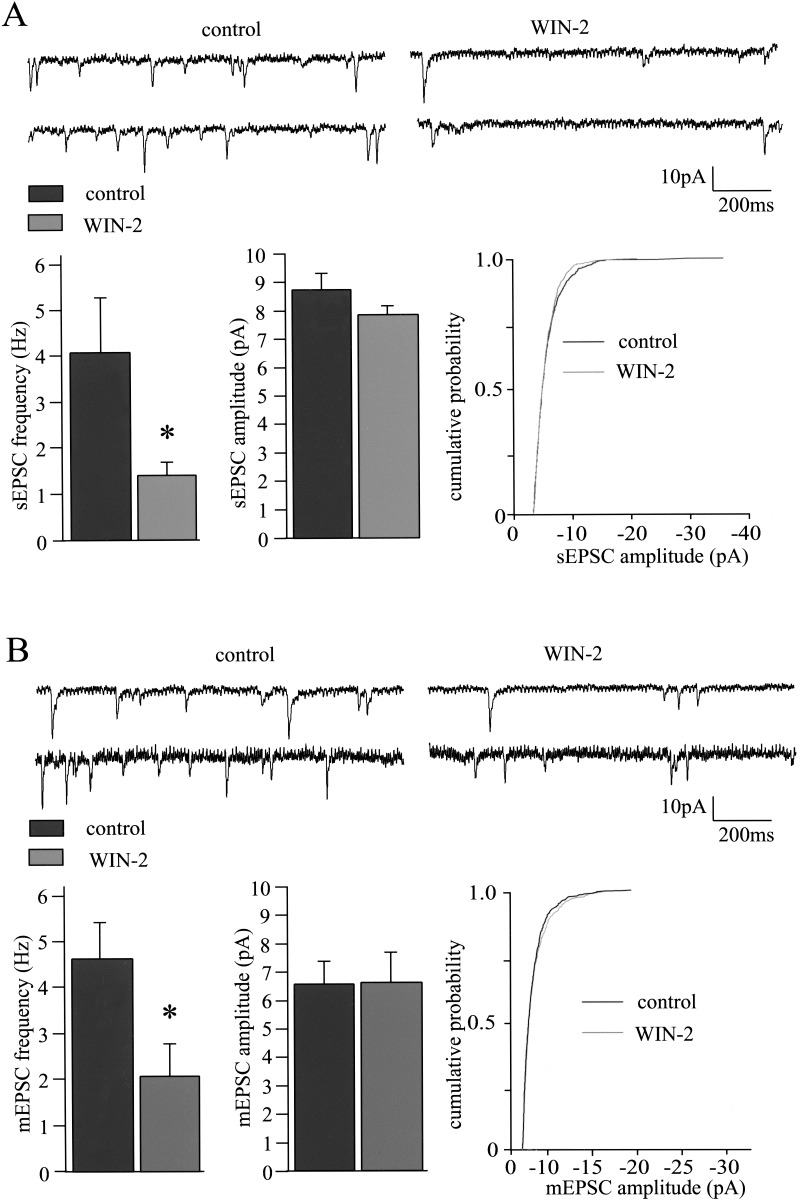
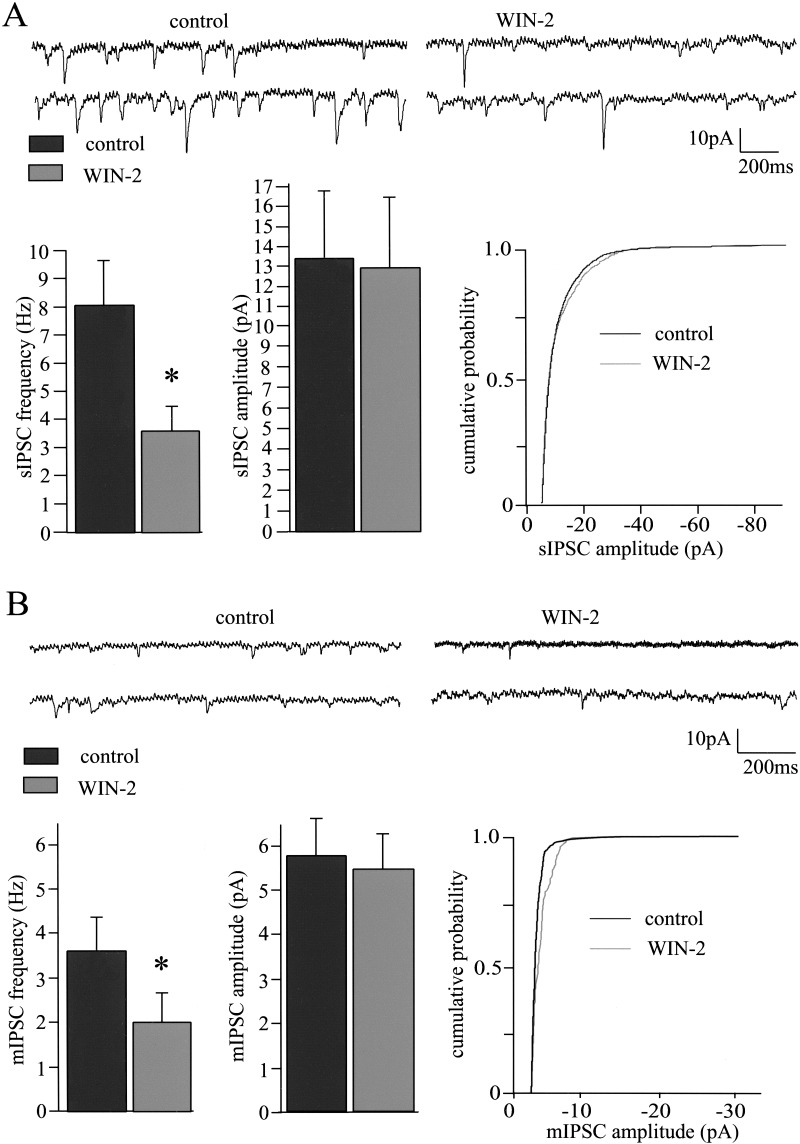
Cannabinoids are assumed to modulate synaptic transmission in the hippocampus (Misner and Sullivan 1999; Hoffman and Lupica 2000), in the nucleus accumbens (Hoffman and Lupica 2001; Robbe et al. 2001) and in the amygdala (Katona et al. 2001) through presynaptic mechanisms. WIN-2-induced increases of the PPR of eEPSCs, AMPA-eEPSCs, and eIPSCs (Figs. 2 and 3) suggest a presynaptic mechanism for the reduction of glutamatergic and GABAergic synaptic transmission in the LA. To further address this issue, we tested whether WIN-2 alters spontaneous (sEPSCs) and action potential-independent miniature (mEPSCs) EPSCs, as well as GABAA receptor-mediated sIPSCs and mIPSCs. WIN-2 (5 μM) significantly reduced the frequencies of sEPSCs and mEPSCs from 4.1 ± 1.1 Hz and 4.5 ± 0.9 Hz to 1.4 ± 0.3 Hz (n = 12; P < 0.05) and 2 ± 0.7 Hz (n = 6; P < 0.05), respectively (Fig. 4), without altering the respective amplitudes (sEPSCs, control, 8.5 ± 0.12 pA; WIN-2, 8.2 ± 0.04 pA; n = 12; P > 0.05; mEPSCs, control, 6.6 ± 0.8 pA; WIN-2, 6.6 ± 1.1 pA; n = 6; P > 0.05). Application of the Na+ channel blocker tetrodotoxin (TTX; 1 μM) reduced the EPSC frequency from 6.1 ± 1.2 Hz to 4.5 ± 0.9 Hz and the EPSC amplitude from 9.8 ± 0.9 pA to 6.6 ± 0.8 pA (data not shown). Under standard conditions with extracellular Ca2+ and Mg2+ concentrations of 2 mM and 1 mM, respectively, WIN-2 did not significantly affect frequencies or amplitudes of sIPSCs and mIPSCs (frequencies: sIPSCs: control, 3.3 ± 0.7 Hz; WIN-2, 2.8 ± 0.8 Hz; n = 4; P > 0.05; mIPSCs: control, 1.3 ± 0.2 Hz; WIN-2, 1.5 ± 0.3 Hz; n = 6; P > 0.05; amplitudes, sIPSCs, control, 10.8 ± 0.9 pA; WIN-2, 11.4 ± 1.4 pA; n = 4; P > 0.05; mIPSCs, control, 4.1 ± 1.6 pA; WIN-2, 4.4 ± 1.8 pA; n = 6; P > 0.05; data not shown). It might be that WIN-2-induced CB1 activation modulates presynaptic release probability only to an extent that is not detectable as a change of sIPSC/mIPSC frequencies with the Ca2+ concentration used. We therefore repeated the experiments with an increased extracellular Ca2+ concentration of 3 mM (with corresponding Mg2+ levels decreased to maintain osmolarity). Under these conditions, the initial sIPCS and mIPSC frequencies were clearly enhanced, and remarkably, WIN-2 now significantly reduced the frequencies of sIPSCs and mIPSCs (sIPSCs: control, 8.0 ± 1.6 Hz; WIN-2, 3.6 ± 0.7 Hz; n = 5; P < 0.05; mIPSCs: control, 3.7 ± 0.6 Hz; WIN-2, 2.0 ± 0.6 Hz; n = 6; P < 0.05), but not the respective amplitudes (sIPSCs: control, 13.3 ± 3.4 pA; WIN-2, 12.8 ± 3.5 pA; n = 5; P > 0.05; mIPSCs: control, 5.8 ± 0.8 pA; WIN-2, 5.4 ± 0.8 pA; n = 6; P > 0.05; Fig. 5).
Figure 4.
Cannabinoids reduce synaptic transmission through presynaptic mechanisms. (A) The CB1 agonist WIN-2 (5 μM) reduces the frequency of sEPSCs from 4.1 ± 1.1 Hz to 1.4 ± 0.3 Hz (n = 12; P < 0.05). A representative pair of traces and the bar diagram (n = 12) are shown. The amplitudes of the sEPSCs are unaffected by WIN-2, as seen in the bars (control, 8.5 ± 0.12 pA; WIN-2, 8.2 ± 0.04 pA; n = 12; P > 0.05) and one representative example of the cumulative probability of sEPSC amplitudes. (B) Application of WIN-2 (5 μM) also reduces the frequency of mEPSCs recorded in the presence of 1 μM TTX (control, 4.5 ± 0.9 Hz; WIN-2, 2 ± 0.7 Hz; n = 6; P < 0.05). A representative pair of traces and the bar diagram are shown. WIN-2 (5 μM) does not alter mean mEPSC amplitude as seen in the bars (control, 6.6 ± 0.8 pA; WIN-2, 6.6 ± 1.1 pA; n = 6; P > 0.05) and one representative example of the cumulative probability of mEPSC amplitudes.
Figure 5.
sIPSCs and mIPSCs recorded in the presence of 3 mM extracellular Ca2+. (A) WIN-2 (5 μM) significantly reduces the frequency of sIPSCs (sIPSCs: control, 8.0 ± 1.6 Hz; WIN-2, 3.6 ± 0.7 Hz; n = 5; P < 0.05). One representative pair of traces and the corresponding bar diagram are depicted. The amplitudes of the sIPSCs are not affected by the agonist, as shown in the bar diagram (control, 13.3 ± 3.4 pA; WIN-2, 12.8 ± 3.5 pA; n = 5; P > 0.05), and one representative example of the cumulative probability of the sIPSC amplitudes. (B) In the presence of 1 μM TTX, WIN-2 (5 μM) had similar effects on mean frequency (control, 3.7 ± 0.6 Hz; WIN-2, 2.0 ± 0.6 Hz; n = 6; P < 0.05) and amplitudes of mIPSCs (control, 5.8 ± 0.8 pA; WIN-2, 5.4 ± 0.8 pA; n = 6; P > 0.05).
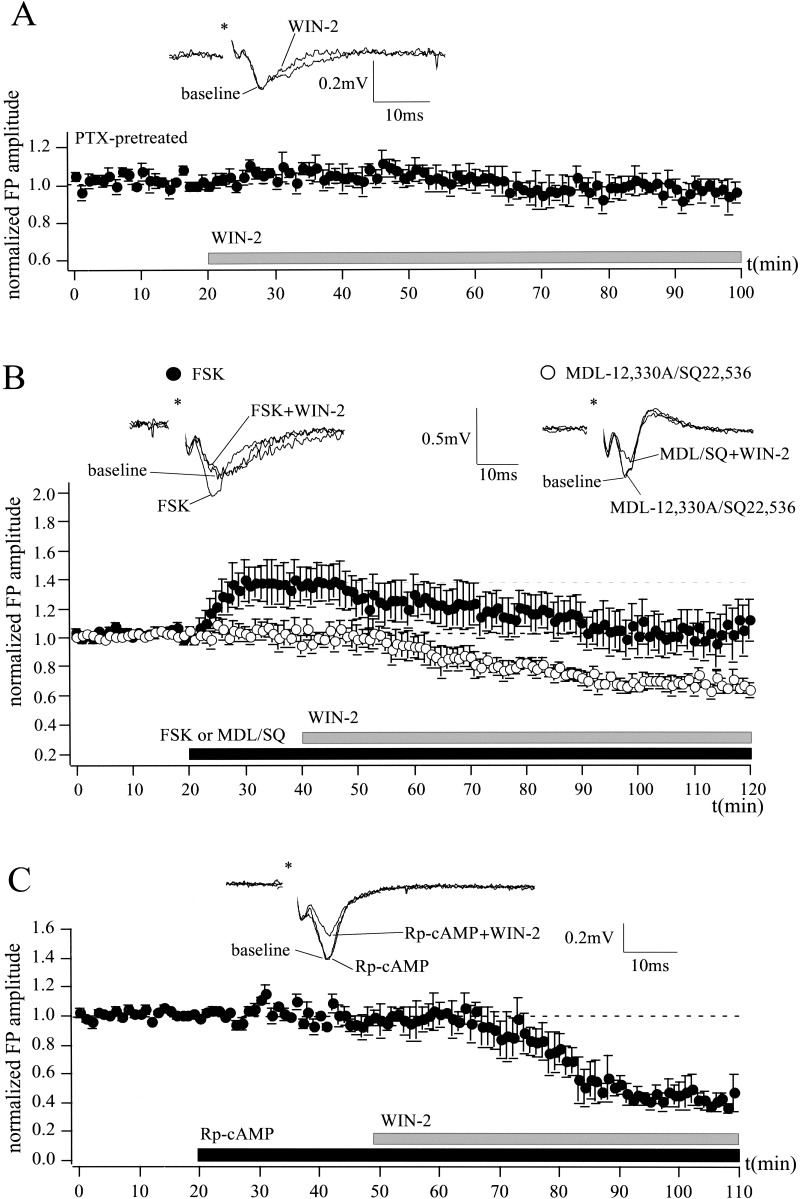
There is evidence that CB1 is coupled to Gi/o proteins and inhibits the AC-PKA pathway (Howlett et al. 1986). To investigate the role of these intracellular mechanisms in the cannabinoid action in the LA, the effect of WIN-2 (5 μM) on FP amplitude was tested in the presence of the Gi/o protein inhibitor pertussis toxin (PTX), the AC activator forskolin (FSK), the AC inhibitors MDL-12,330A and SQ 22,536, and the protein kinase A (PKA) inhibitor Rp-cAMP (Fig. 6). Preincubation of the slices in PTX (5 μg/mL) for 5–7 h abolished the effect of WIN-2 (5 μM) on FP amplitude to 95 ± 6% of baseline (n = 6; P > 0.05; Fig. 6A). In contrast to the AC inhibitors, MDL-12,330A (10 μM; n = 4; P > 0.05) and SQ 22,536 (50 μM; n = 3; P > 0.05), which did not affect the FP amplitude, the AC activator FSK (10 μM) rapidly increased FP amplitude to 139 ± 12% of baseline (n = 7; P > 0.05; Fig. 6B). In control experiments, this effect of FSK was stable for at least 60 min (data not shown). Neither activation of the AC by FSK (FSK, 100%; FSK + WIN-2, 66 ± 9%; n = 7; P < 0.05) nor inhibition of the AC by MDL-12,330A or SQ 22,536 (MDL/SQ, 100%; MDL/SQ + WIN-2, 63 ± 6%; n = 7; P < 0.05) prevented the cannabinoid-induced reduction of the FP amplitude. Furthermore, the WIN-2-induced decrease of synaptic transmission was not inhibited by the PKA inhibitor Rp-cAMP (25 μM; Rp-cAMP, 100%; Rp-cAMP + WIN-2, 49 ± 10%; n = 4; P < 0.05; Fig. 6C). These results indicate a minor role of the AC-PKA pathway in cannabinoid actions in the LA.
Figure 6.
Cannabinoid action in the LA involves the activation of Gi/o proteins, but not the inhibition of the AC-PKA pathway. (A) Preincubation of slices with the Gi/o protein inhibitor PTX (5 μg/mL) for 5–7 h abolishes the effects of WIN-2 (5 μM) on extracellularly recorded FP amplitudes (WIN-2, 95 ± 6% of baseline; n = 6; P > 0.05). (B) Application of the AC activator FSK (10 μM), but not of the AC inhibitors MDL-12,330A (10 μM) and SQ 22,536 (50 μM), rapidly increases FP amplitude to 139 ± 12% of baseline (n = 7; P > 0.05). Neither the AC activator (FSK, 100%; FSK + WIN-2, 66 ± 9%; n = 7; P < 0.05), nor the AC inhibitors (MDL/SQ, 100%; MDL/SQ + WIN-2, 63 ± 6%; n = 7; P < 0.05) alter WIN-2-induced reduction of FP amplitude. (C) Inhibition of the PKA by Rp-cAMP (25 μM) does not prevent WIN-2 action on synaptic transmission (Rp-cAMP, 100%; Rp-cAMP + WIN-2, 49 ± 10%; n = 4; P < 0.05). Representative traces are shown. All data are normalized to the respective baseline values (last 10 min of baseline). Asterisks mark stimulation artifacts.
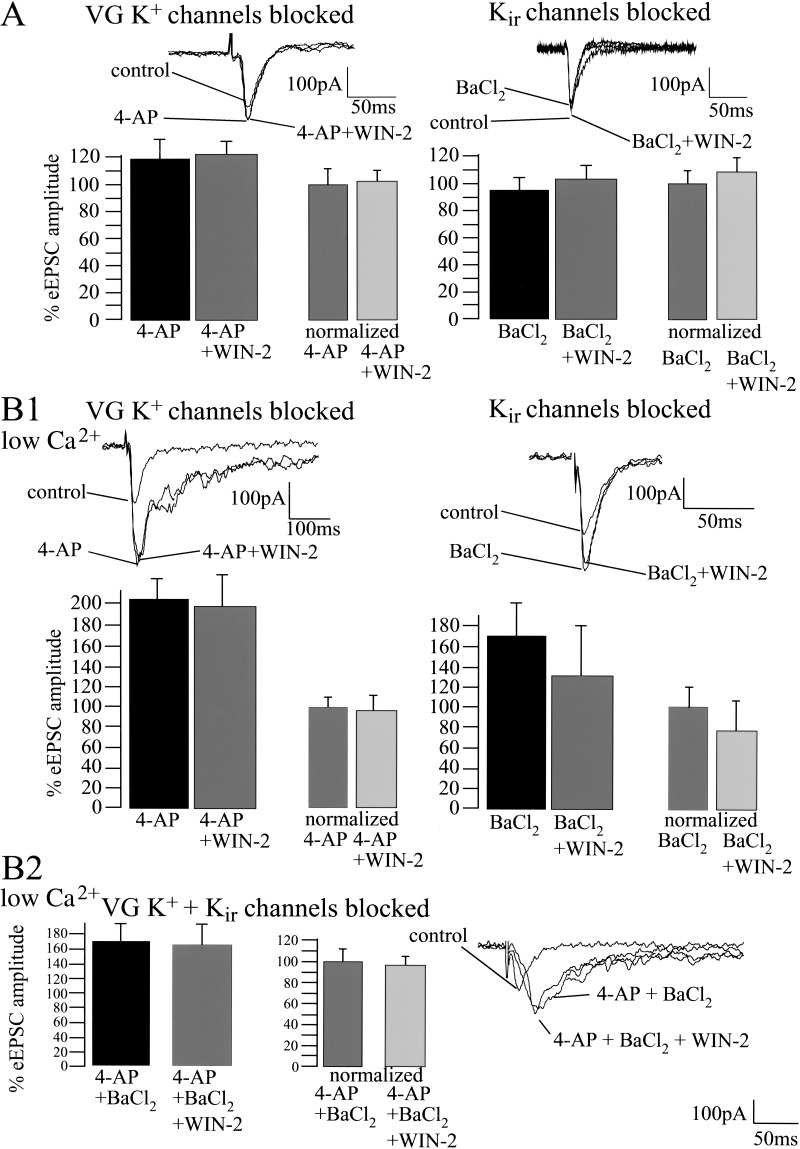
It has been shown previously that cannabinoids modulate voltage-dependent and voltage-independent K+ channels in hippocampal neurons and cells transfected with CB1 (Deadwyler et al. 1995; Mackie et al. 1995; McAllister et al. 1999; Schweitzer 2000). To evaluate a possible cannabinoid-induced modulation of K+ channels in the amygdala, we measured the effect of WIN-2 (5 μM) on eEPSC amplitude in the presence of 4-AP (100 μM), a blocker of voltage-dependent K+ channels (Mathie et al. 1998; Bordey and Sontheimer 1999), and BaCl2 (300 μM), a blocker of G protein-gated inwardly rectifying K+ channels (Kir; Coetzee et al. 1999; McAllister et al. 1999; Takigawa and Alzheimer 1999). Figure 7A shows that 4-AP (control, 100%; 4-AP, 119 ± 14%; n = 5; P > 0.05) and BaCl2 (control, 100%; BaCl2; 96 ± 6%; n = 6; P > 0.05) did not significantly affect the amplitude of the eEPSCs. However, both 4-AP and BaCl2 clearly abolished the WIN-2-induced decrease of eEPSC amplitude (4-AP, 100%; 4-AP + WIN-2, 103 ± 10%; n = 5; P > 0.05; BaCl2, 100%; BaCl2 + WIN-2, 108 ± 8%; n = 5; P > 0.05). However, the validity of these results might be limited by the fact that K+ channel blockers can prolong the presynaptic action potential by diminishing repolarization of the presynaptic terminal, thus enhancing Ca2+ influx and saturating neurotransmitter release (Hoffman and Lupica 2000). We therefore performed additional experiments investigating the effects of 4-AP and BaCl2 on WIN-2-induced modulation of synaptic transmission after decreasing extracellular Ca2+ concentration from 2 to 0.5 mM (Mg2+ concentration increased from 1 to 2.5 mM to obtain equal osmolarity). Reducing extracellular Ca2+ decreased the eEPSC amplitude to 55 ± 9% (n = 12; P < 0.05; data not shown). For the further steps of the experiments, the obtained values were set to 100%. The following application of the K+ channel blockers increased eEPSC amplitude markedly (control, 100%; 4-AP, 201 ± 20%; n = 4; P < 0.05; control, 100%; BaCl2, 169 ± 26%; n = 4; P < 0.05; Fig. 7B1). In addition, under the condition of reduced Ca2+ concentration, both channel blockers inhibited the effects of subsequently applied WIN-2 on synaptic transmission (4-AP, 100%; 4-AP + WIN-2, 94 ± 15%; n = 4; P > 0.05; BaCl2, 100%; BaCl2 + WIN-2, 78 ± 28%; n = 4; P > 0.05). Similar results were obtained when both 4-AP and BaCl2 were applied together (control, 100%; 4-AP + BaCl2, 168 ± 23%; n = 4; P < 0.05; 4-AP + BaCl2, 100%; 4-AP + BaCl2 + WIN-2, 98 ± 22%; n = 4; P > 0.05; Fig. 7B2).
Figure 7.
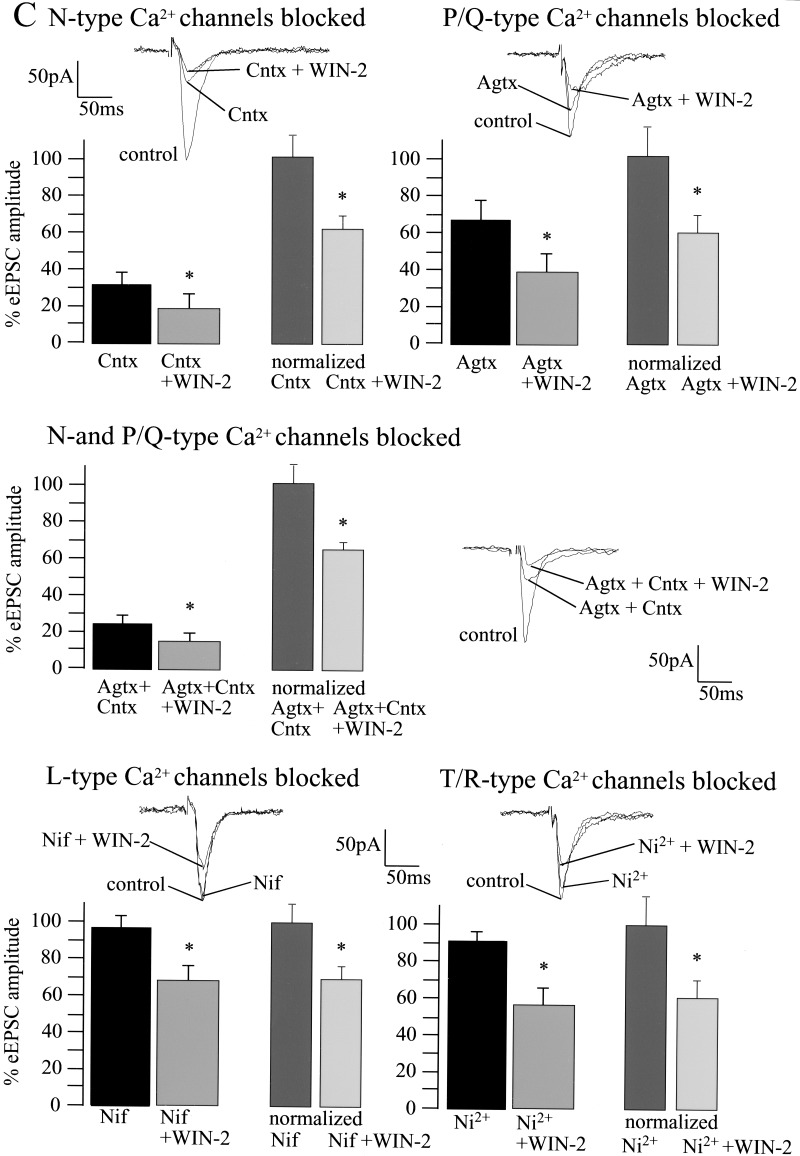
Cannabinoid-induced decrease of basal synaptic transmission involves the modulation of voltage-sensitive and inwardly rectifying K+ channels, but is unaffected by inactivation of pre- and postsynaptic Ca2+ channels. (A) Whole-cell recordings reveal that 4-AP (100 μM; control, 100%; 4-AP, 119 ± 14%; n = 5; P > 0.05) and BaCl2 (control, 100%; BaCl2, 96 ± 6%; n = 6; P > 0.05) slightly affect basal synaptic transmission. Both 4-AP and BaCl2 inhibit the effect of WIN-2 (5 μM) on eEPSC amplitude (4-AP, 100%; 4-AP + WIN-2, 103 ± 10%; n = 5; P > 0.05; BaCl2, 100%; BaCl2 + WIN-2, 108 ± 8%; n = 5; P > 0.05). (B1,B2) Under conditions in which the extracellular Ca2+ concentration is decreased to 0.5 mM, both K+ channel blockers increase eEPSC amplitudes markedly (baseline, 100%; 4-AP, 201 ± 20%; n = 4; P < 0.05; baseline, 100%; BaCl2, 169 ± 26%; n = 4; P < 0.05) and inhibit WIN-2 action on synaptic transmission (4-AP, 100%; 4-AP + WIN-2, 94 ± 15%; n = 4; P > 0.05; BaCl2, 100%; BaCl2 + WIN-2, 78 ± 28%; n = 4; P > 0.05). Similar results are obtained when both 4-AP and BaCl2 are applied together (baseline, 100%; 4-AP + BaCl2, 168 ± 23%; n = 4; P < 0.05; 4-AP + BaCl2, 100%; 4-AP + BaCl2 + WIN-2, 98 ± 22%; n = 4; P > 0.05). (C) Blockade of presynaptic N- and P/Q- type Ca2+ channels with ω-Conotoxin (Cntx; 1 μM) and ω-Agatoxin (Agtx; 200 nM) reduce eEPSC amplitudes to 31 ± 6% (n = 4; P < 0.05) and 66 ± 11% (n = 5; P = 0.05), respectively. Blockade of postsynaptic L- and R/T-type Ca2+ channels with Nifedipine 20 μM (control, 100%; Nifedipine, 94 ± 6%; n = 4; P > 0.05) and Ni2+ 50 μM (control, 100%; Ni2+, 90 ± 5%; n = 5; P > 0.05) does not affect eEPSC amplitude. Neither pre- nor postsynaptic Ca2+ channel blockers affect the WIN-2-induced decrease of the eEPSC amplitude (Cntx, 100%; Cntx + WIN-2, 60 ± 8%; n = 5; P < 0.05; Agtx, 100%; Agtx + WIN-2, 59 ± 11%; n = 5; P < 0.05; Cntx + Agtx, 100%; Cntx + Agtx + WIN-2, 67 ± 7%; n = 4; P < 0.05; Nifedipine, 100%; Nifedipine + WIN-2, 66 ± 10%; n = 5; P < 0.05; Ni2+, 100%; Ni2+ + WIN-2, 63 ± 9%; n = 5; P < 0.05). All bars reflect mean ± SEM.
Cannabinoids also inhibit presynaptic voltage-dependent Ca2+ channels (Hoffman and Lupica 2000). The effects of WIN-2 on eEPSCs in the LA were therefore tested while blocking presynaptic Ca2+ entry through N- and P/Q-type channels by nonoverlapping concentrations of ω-Conotoxin (Cntx; 1 μM) and ω-Agatoxin (Agtx; 200 nM; Sidach and Mintz 2000), respectively (Fig. 7C). Postsynaptic L-type Ca2+ channels were antagonized by Nifedipine (20 μM) and post/presynaptic T/R-type Ca2+ channels by Ni2+ (50 μM). Cntx and Agtx reduced eEPSC amplitudes to 31 ± 6% (n = 4; P < 0.05) and 66 ± 11% (n = 5; P = 0.05) of baseline, respectively. However, neither substance affected the WIN-2-induced inhibition of synaptic transmission (Cntx, 100%; Cntx + WIN-2, 60 ± 8%; n = 5; P < 0.05; Agtx, 100%; Agtx + WIN-2, 59 ± 11%; n = 5; P < 0.05). Furthermore, the WIN-2 effect was still present when N- and P/Q-type channels were simultaneously blocked by Cntx and Agtx (baseline, 100%; Cntx + Agtx, 27 ± 9%; n = 4; P < 0.05; Cntx + Agtx, 100%; Cntx + Agtx + WIN-2, 67 ± 7%; n = 4; P < 0.05). At the concentrations used, the L- and R/T-type channel blockers Nifedipine and Ni2+ slightly decreased basal synaptic transmission (Nifedipine, 94 ± 6% of baseline; n = 4; P > 0.05; Ni2+, 90 ± 5% of baseline; n = 5; P > 0.05), but did not affect cannabinoid actions (Nifedipine, 100%; Nifedipine + WIN-2, 66 ± 10%; n = 5; P < 0.05; Ni2+, 100%; Ni2+ + WIN-2, 63 ± 9%; n = 5; P < 0.05).
DISCUSSION
CB1 Activation Decreases Synaptic Transmission in the LA Through Activation of Gi/o Proteins and Modulation of K+ Conductances
Cannabinoids are known to inhibit stimulus-evoked synaptic transmission in several brain regions such as hippocampus (Misner and Sullivan 1999), nucleus accumbens (Hoffman and Lupica 2001; Robbe et al. 2001) and prefrontal cortex (Auclair et al. 2000). The present investigation shows that pharmacological activation of CB1 decreases basal synaptic transmission reflected in a decrease in FP and eEPSC amplitudes in the LA. Several investigations have revealed that the activation of Gi/o proteins (Mackie and Hille 1992; Misner and Sullivan 1999), and the subsequent inhibition of the AC-PKA cascade (Howlett et al. 1986), play major roles in cannabinoid-induced effects. There is evidence that cannabinoid receptors can also couple to Gs proteins (Glass and Felder 1997) and stimulate the AC-PKA pathway under certain conditions (Maneuf and Brotchie 1997). Our results indicate that, in the LA, the cannabinoid-induced decrease of basal synaptic transmission requires the activation of Gi/o proteins. However, interfering with AC or PKA activity did not affect the WIN-2-induced effects, indicating a minor role of the AC-PKA pathway in cannabinoid-induced decrease of synaptic transmission. In fact, cannabinoid actions have also been found to be independent of AC in the mouse nucleus accumbens (Robbe et al. 2001). The inhibition of presynaptic voltage-dependent Ca2+ channels (Mackie and Hille 1992; Twitchell et al. 1997; Hoffmann and Lupica 2000), and the modulation of voltage-dependent and voltage-independent K+ channels (Deadwyler et al. 1995; Mackie et al. 1995; McAllister et al. 1999; Schweitzer 2000) are established mechanisms involved in cannabinoid action. Surprisingly, in the present study, neither blockade of presynaptic N- and P/Q-, nor of postsynaptic L- and R/T-type voltage-gated Ca2+ channels altered the WIN-2-induced decrease of eEPSCs, indicating a minor role of Ca2+ channels in cannabinoid effects on basal synaptic transmission in the LA. In contrast, blockade of voltage-dependent K+ channels and blockade of G protein-gated Kir channels inhibited the cannabinoid action in the LA. This was the case under the conditions of regular (2 mM), as well as decreased (0.5 mM) extracellular Ca2+ concentrations, the latter of which should be able to eliminate the problem of a possible saturation of neurotransmitter release due to enhanced Ca2+ influx after K+ channel blockade. These findings underline the importance of K+ channel modulation for cannabinoid actions in the LA and resemble those obtained in the mouse nucleus accumbens (Robbe et al. 2001). In contrast, in the hippocampus, the modulation of voltage-gated Ca2+ channels (Sullivan 1999; Hoffman and Lupica 2000), but not of K+ channels (Hoffman and Lupica 2000) was shown to be important for the effects of cannabinoids. It is apparent from these results that cannabinoid actions vary between different brain regions (for review, see Wilson and Nicoll 2002).
Cannabinoids Decrease Isolated Glutamatergic and GABAergic Synaptic Transmission in the LA Through Activation of CB1
FPs and postsynaptic currents are mixed excitatory/inhibitory synaptic responses. It has been shown that cannabinoids, in most structures studied so far, reduce inhibitory GABAergic synaptic transmission through activation of CB1 (Hajos et al. 2001; Katona et al. 2001). However, the role of CB1 in cannabinoid-induced reduction of glutamatergic synaptic transmission appears to be less clear (Misner and Sullivan 1999; Auclair et al. 2000; Hoffman and Lupica 2001; Robbe et al. 2001; Gerdeman et al., 2002; for review, see Wilson and Nicoll 2002). In the present study, we found that WIN-2 significantly decreases the amplitudes of both isolated AMPA-eEPSCs and eIPSCs in the LA of wild-type mice, but not in animals lacking CB1. These results indicate that cannabinoids can decrease glutamatergic, as well as GABAergic synaptic transmission in this structure via an activation of CB1. In the lateral amygdala of the rat and mouse, the vast majority of CB1 is expressed on presynaptic terminals of GABAergic cells, whereas nearly all boutons forming asymmetrical, presumably glutamatergic synapses were found to be devoid of CB1 (Katona et al. 2001). However, it has been shown that CB1 mRNA is also present in many non-GABAergic cells probably projecting glutamatergic neurons (Marsicano and Lutz 1999).
Since in the present investigation WIN-2 application reduces GABAergic synaptic transmission faster than glutamatergic synaptic transmission; one could expect that FP amplitude should increase soon after cannabinoid application, although the exact contribution of either form of synaptic transmission to the final FP is unclear. However, we did not find such an effect (Fig. 1). This is in accordance with our finding that WIN-2 application decreases FP amplitude despite its effects on both excitatory and inhibitory synaptic transmission, which would also be expected to compensate each other. Together, these results indicate that, in the LA, cannabinoid actions on glutamatergic synaptic transmission override those on GABAergic synaptic transmission, at least under the given experimental conditions, thus leading to an overall decrease of excitability.
In our study, WIN-2 induced an increase of the PPRs of eEPSCs and AMPA-eEPSCs together with a significant decrease of sEPSC and mEPSC frequencies. These findings speak in favor of an inhibition of glutamatergic synaptic transmission in the LA through presynaptically located CB1. This is consistent with findings in various other brain regions, in which a presynaptic site of CB1 action is also suggested (Misner and Sullivan 1999; Auclair et al. 2000). WIN-2 also increased PPR of eIPSCs, but was not able to significantly affect the frequencies of sIPSCs and mIPSCs under standard conditions with 2 mM extracellular Ca2+. A lack of cannabinoid-induced decrease of mIPSCs frequencies was also described in the basolateral complex of the amygdala (Katona et al. 2001). In this study, only the frequencies of sIPSCs were reduced by WIN-2. However, in the present investigation, WIN-2 exerted significant effects on the frequencies, but not the amplitudes of sIPSCs and mIPSCs when extracellular Ca2+ was enhanced to 3 mM. The question arises, why an enhanced Ca2+ concentration is required for the effects of WIN-2 on sIPSCs and mIPSCs, but not on eIPSCs. There might be the following explanation: Stimulation of afferents during eIPSC recording induces the influx of a certain amount of Ca2+ to evoke transmitter release, which is certainly lower when measuring sIPSCs and mIPSCs without afferent stimulation. Our experiments show that the WIN-2-induced reduction of synaptic transmission is caused by a Gi/o protein-mediated modulation of K+ but not of Ca2+ conductances. It might be that WIN-2-induced CB1 activation modulates K+ conductances only to an extent that is not detectable as a change of transmitter release on the level of sIPSCs/mIPSCs with the standard Ca2+ concentration used. In contrast, the Ca2+ influx during afferent stimulation is much higher than during measurement of sIPSCs/mIPSCs. Therefore, we were able to detect an effect of WIN-2 on synaptic transmission, but not on sIPSC/mIPSC frequencies. However, increasing extracellular Ca2+ concentration during sIPSC/mIPSC measurement obviously increased Ca2+ influx toward a level reached by stimulation of afferents, thus allowing us to detect WIN-2-induced changes of transmitter release. The increase of PPR of eIPSCs, the WIN-2-induced decrease of sIPSC and mIPSC frequencies, and the lack of an effect on sIPSC/mIPSC amplitudes observed in the present study together with the immunocytochemical data showing that CB1 is expressed on presynaptic terminals of GABAergic synapses in the amygdala (Katona et al. 2001) provide evidence for a presynaptic mechanism for the cannabinoid-induced decrease of GABAergic synaptic transmission.
The present results indicate that cannabinoids control synaptic transmission in the LA by modulating both glutamatergic and GABAergic synapses via presynaptic mechanisms. In a recent investigation, we revealed that the endogenous cannabinoid system in the amygdala plays a major role in the extinction of aversive memories (Marsicano et al. 2002). In addition, various studies have implicated the amygdala in the processing and modulation of pain (Manning et al. 2001; Neugebauer and Li 2002), particularly in chronic pain states (Paulson et al. 2002), which are considerably influenced by emotional components and aversive memories in humans (Villemure and Bushnell 2002). Thus, cannabinoid-mediated modulation of synaptic processes in the amygdala might be a potential novel therapeutic target.
MATERIALS AND METHODS
Slice Preparation
Male C57Bl6/JOlaHsd mice (42–63 d, Harlan Winkelmann) and male CB1−/− and CB1+/+ littermates (49–60 d) were used for the investigations. CB1−/− and CB1+/+ mice were generated as described by Marsicano et al. (2002). For slice preparation, the animals were anesthetized with isoflurane and decapitated. Slices were prepared during the light phase. The brains were removed rapidly and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in millimolar) NaCl 125, KCl 2.5, NaHCO3 25, CaCl2 2, MgCl2 1, D-glucose 25, NaH2PO4 1.25 (pH 7.4), and bubbled with a 95% O2/5% CO2 mixture. Coronal slices of the amygdala (400-μm thick) were prepared using a vibroslicer (FTB). After incubation in a holding chamber with ACSF (22–25°C) for at least 60 min, the slices were placed in the recording chamber of the setup and superfused with ACSF at a flow rate of 1.5 mL/min.
Electrophysiology
Square pulse stimuli (0.066 Hz, 5–15 V, 200 μsec) were delivered via bipolar concentric tungsten electrodes insulated to the tip (50-μm tip diameter), and positioned on the border between the LA and the external capsule to evoke eEPSCs, eIPSCs, and FPs.
All recordings were performed in the LA. All experiments were performed at room temperature (22–25°C). FPs were recorded using glass microelectrodes (1–2 MΩ) filled with ACSF. The stimulus intensities were adjusted in a manner to produce a FP of ∼50% of the maximum amplitude. The voltage differences between the sharp negative onset and the negative peak (a), and between the negative peak and the succeeding positive peak (b), were measured, and the amplitudes of the FPs were calculated as (a+b)/2.
For whole-cell patch-clamp recordings, principal neurons of the LA were visualized using infrared video-microscopy and the gradient contrast system (Zeiss). For technical details, see Dodt et al. (2002). For recording of eEPSCs, glass electrodes (4.5–5 MΩ) contained (in millimolar) K-D-gluconat 130, KCl 5, Mg-ATP 2, D-glucose 10, EGTA 0.5, HEPES 10 (pH 7.4). Currents were recorded using a switched voltage-clamp amplifier (SEC-10L; npi Electronics) with switching frequencies of 75–80 kHz (25% duty cycle). Series resistance was monitored continuously and compensated in bridge mode (for details, see Swandulla and Misgeld 1990). Neuronal input resistance was monitored by injecting hyperpolarizing current pulses (300 msec, −10 mV, 0.066 Hz) through the patch electrode. All patch-clamp experiments were performed at a holding potential of −70 mV.
Isolated AMPA receptor-mediated eEPSCs were measured in the presence of 50 μM D-(-)-2-amino-5-phosphonopentanoic acid (D-AP5), 50 μM picrotoxin, and 200 μM 3-aminopropyl (diethoxymethyl)phosphinic acid (CGP 35348). Evoked GABAA receptor-mediated synaptic transmission was isolated by application of 50 μM D-AP5 and 5 μM 2,3-dioxo-6-nitro-1,2,3,4-tetrahydro-benzo[f]quinoxaline-7-sulfonamide (NBQX) and 200 μM CGP 35348. The pipettes were filled with a solution containing (in millimolar) Mg-ATP 2, CsCH3SO3 100, CsCl 60, EGTA 0.2, HEPES 10, MgCl2 1, QX314 5, and Na3GTP 0.3 (pH 7.3). Evoked GABAA receptor-mediated currents at −70 mV holding potential were measured as Cl− inward currents and, hence, defined as eIPSCs. Paired-pulse stimulation was performed by delivering the same stimulus at 50 msec inter-pulse intervals. PPR was determined by dividing the second amplitude by the first one (eEPSC2/eEPSC1; AMPA-eEPSC2/AMPA-eEPSC1; eIPSC2/eIPSC1).
All recordings were amplified, filtered (3 kHz), and digitized (9 kHz). The digitized data were stored to a Power Macintosh G3 computer by a data acquisition and evaluation program (Pulse v. 8.5; Heka Electronic).
The amplitudes and frequencies of sEPSCs and mEPSCs were studied by continuous recording over 300 sec without, or in the presence of 1 μM TTX, respectively. The peak amplitudes of the sEPSCs and mEPSCs were measured off-line automatically using a customized event detection macro (Igor 4.0, Wave Metrics Inc.) with an adjustable amplitude threshold. Frequencies were calculated by dividing the total number of EPSCs by the total time sampled.
Statistical analysis was carried out using the paired Student's t test to compare the values of 10 min of a stable baseline and after application of the different substances, respectively. The latter were obtained by choosing the values as soon as the substance application led to stable effects for at least 10 min. The Kolmogorov-Smirnov test (StatView 5.0, SAS Institute) was used for comparison of the amplitude distributions of sEPSCs and mEPSCs. In both tests, P < 0.05 was considered as a significant difference. Data are presented as mean ± SEM. All electrophysiological experiments were performed in accordance with the guidelines of the Ethical Committee on the Use and Care of Animals (Government of Bavaria, Germany).
Chemicals
Drugs were applied via the superfusion system. The following pharmacological compounds were used: WIN 55,212-2, pertussis toxin, forskolin (7β-Acetoxy-1α, 6β, 9α-trihydroxy-8, 13-epoxy-labd-14-en-11-one), MDL-12,330A (cis-N-[2-phenylcyclo-pentyl]-azacyclotridec-1-en-2-amine), SQ 22,536 (9-[tetrahydro-2-furanyl]-9H-purin-6-amine), Rp-cAMP (8-Bromoadenosine-3‵,5‵-cyclic monophosphorothioate), NBQX, D-AP5, picrotoxin, BaCl2, 4-aminopyridine, and Nifedipine from RBI/Sigma (RBI), tetrodotoxin, CGP 35348, ω-Agatoxin IVA and ω-Conotoxin GVIA (Tocris, Biotrend), SR 141716A (NIMH Chemical Synthesis and Drug Supply Program). Stock solutions of WIN 55,212-2 (10 mM), SR 141716A (10 mM) and Nifedipine (50 mM) were prepared in DMSO and stored at −20°C. Final DMSO concentrations were ≤0.05%. Before all experiments, fatty acid free BSA (1 mg/mL) was rinsed through the system to avoid binding of WIN 55,212-2 and SR 141716A to the walls of the tubing.
As the WIN-2 concentrations applied in experiments using slice preparations vary between 1 and 10 μM throughout the literature, we performed a dose finding study (1–2.5–5–10 μM WIN-2) in the LA of C57BL/6JOlaHsd mice (data not published), which revealed a submaximal concentration of 5 μM in FP, eEPSC, and eIPSC measurements. We therefore choose this concentration for the present study.
Acknowledgments
We thank Christine Hilf for excellent technical assistance and Barbara Wölfel for breeding and genotyping of CB1-deficient mice, Ursula Golbs for secretarial work and Dr. C. Wotjak for critical reading of the manuscript and valuable considerations. S.C.A. thanks Dr. A. Beyer, Prof. Dr. Dr. h.c. K. Peter, and the Claussen-Simon Stiftung des Stifterverbandes der Deutschen Wissenschaft for supporting her scientific work.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL azad@mpipsykl.mpg.de; FAX 49-89-30622-402.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.53303.
REFERENCES
- Asztely F, Xiao MY, Gustafsson B. Long-term potentiation and paired-pulse facilitation in the hippocampal CA1 region. NeuroReport. 1996;7:1609–1612. doi: 10.1097/00001756-199607080-00016. [DOI] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Differential inhibition of glial K+currents by 4-AP. J Neurophysiol. 1999;82:3476–3487. doi: 10.1152/jn.1999.82.6.3476. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. The functional neuroanatomy of brain cannabinoid receptors. Neurobiol Dis. 1998;5:417–431. doi: 10.1006/nbdi.1998.0229. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, et al. Molecular diversity of K+-channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- Dodt HU, Eder M, Schierloh A, Zieglgänsberger W. Infrared-guided laser stimulation of neurons in brain slices. Sci STKE. 2002;120:PL2. doi: 10.1126/stke.2002.120.pl2. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAAsynaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: A comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium-channels in neuroblastoma-glioma cells. Proc Natl Acad Sci. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: Effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM. Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br J Pharmacol. 1997;120:1397–1398. doi: 10.1038/sj.bjp.0701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Merin NM, Meng ID, Amaral DG. Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J Neurosci. 2001;21:8238–8246. doi: 10.1523/JNEUROSCI.21-20-08238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–R5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res. 1999;822:237–242. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- Mathie A, Wooltorton JRA, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Griffin G, Satin LS, Abood ME. Cannabinoid receptors can activate and inhibit G protein-coupled inwardly rectifying potassium-channels in a Xenopusoocyte expression system. J Pharmacol Exp Ther. 1999;291:618–626. [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: High concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol. 2002;87:103–112. doi: 10.1152/jn.00264.2001. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Casey KL, Morrow TJ. Long-term changes in behavior and regional cerebral blood flow associated with painful peripheral mononeuropathy in the rat. Pain. 2002;95:31–40. doi: 10.1016/s0304-3959(01)00370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: Unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Schweitzer P. Cannabinoids decrease the K+M-current in hippocampal CA1 neurons. J Neurosci. 2000;20:51–58. doi: 10.1523/JNEUROSCI.20-01-00051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidach SS, Mintz IM. Low-affinity blockade of neuronal N-type Ca2+-channels by the spider toxin θ-agatoxin-IVA. J Neurosci. 2000;20:7174–7182. doi: 10.1523/JNEUROSCI.20-19-07174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Swandulla D, Misgeld U. Development and properties of synaptic mechanisms in a network of rat hypothalamic neurons grown in culture. J Neurophysiol. 1990;64:715–726. doi: 10.1152/jn.1990.64.3.715. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. G protein-activated inwardly rectifying K+(GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol. 1999;517:385–390. doi: 10.1111/j.1469-7793.1999.0385t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium-channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- ————— Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]