Abstract
For β-d-glucosylisophosphoramide mustard (β-d-Glc-IPM), a new alkylating drug in which isophosphoramide mustard is stabilized, a higher selectivity and lower myelotoxicity was observed than for the currently used cytostatic ifosfamide. Because β-d-Glc-IPM is hydrophilic and does not diffuse passively through the lipid bilayer, we investigated whether a transporter may be involved in the cellular uptake. A variety of cloned Na+-sugar cotransporters were expressed in Xenopus oocytes, and uptake measurements were performed. By tracer uptake and electrical measurements it was found that β-d-Glc-IPM was transported by the low-affinity Na+-d-glucose cotransporter SAAT1, which had been cloned from pig and is also expressed in humans. At membrane potentials between −50 and −150 mV, a 10-fold higher substrate affinity (Km ≈ 0.25 mM) and a 10-fold lower Vmax value were estimated for β-d-Glc-IPM transport than for the transport of d-glucose or methyl-α-d-glucopyranoside (AMG). Transport of β-d-Glc-IPM and glucose by SAAT1 is apparently performed by the same mechanism because similar sodium dependence, dependence on membrane potential, electrogenicity, and phlorizin inhibition were determined for β-d-Glc-IPM, d-glucose, and AMG. Transcription of human SAAT1 was demonstrated in various human carcinomas and tumor cell lines. In one of these, the human carcinoma cell line T84, phlorizin inhibitable uptake of β-d-Glc-IPM was demonstrated with substrate saturation and an apparent Km of 0.4 mM. The data suggest that the Na+-d-glucose cotransporter SAAT1 transports β-d-Glc-IPM into human tumor cells and may accumulate the drug in the cells. They provide an example for drug targeting by employing a plasma membrane transporter.
In recent years progress in cancer chemotherapy has been slow. Despite numerous efforts to improve the situation of the patients, no breakthrough could be achieved. Because the pronounced side effects of chemotherapeutic treatment are often dose limiting, new concepts for drug targeting are required (1). In 1986 one of us (M.W.) started a program to study carrier systems for drugs. At variance to a series of other reports where monosaccharides were used as carriers (2, 3), M.W. decided to connect therapeutically active compounds to the 1-position of the carbohydrate skeleton (4). Thereby hydrophilic conjugates were created that could release the drugs by hydrolysis. Following this concept, isophosphoramide mustard, which represents the therapeutically active metabolite of ifosfamide, was selected for conjugation. Ifosfamide itself, which is used extensively in the clinics, needs cytochrome P450 activation, mainly in the liver, to be therapeutically active. For reasons of chemical stability and apparent therapeutic efficiency the d-glucose conjugate β-d-glucosylisophosphoramide mustard (β-d-Glc-IPM) was selected for further development. β-d-Glc-IPM shows a lower myelotoxicity and a higher anti-tumor activity than ifosfamide as demonstrated with cultured cells and with human tumors grown in immunodeficient mice (4). Meanwhile, a phase 1 clinical study has been started. Because some protective effect of phlorizin against the cytotoxicity of β-d-Glc-IPM was observed in murine leukemia L1210 cells (4) we wondered whether one of the phlorizin-inhibitable Na+-sugar cotransporters of the SGLT family is involved in cellular uptake of β-d-Glc-IPM (5). We expressed the functionally active Na+-sugar cotransporters of this family that have been cloned from rabbit, pig, dog, and human, and tested them for phlorizin-inhibitable transport of β-d-Glc-IPM. It could be demonstrated that the low-affinity Na+-d-glucose cotransporter SAAT1 from pig (6, 7) translocated β-d-Glc-IPM with a higher affinity than d-glucose. A cDNA fragment of a prospective human SAAT1 subtype was detected in different human tumors and tumor cell lines, and phlorizin-inhibitable β-d-Glc-IPM uptake was demonstrated in one of these cell lines.
MATERIALS AND METHODS
Chemicals.
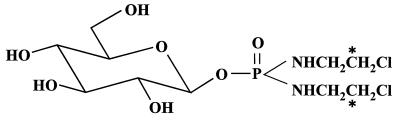
The synthesis of β-d-glucosyl[14C]isophosphoramide mustard ([14C]β-d-Glc-IPM) and the unlabeled compound was performed by identical procedures. Fig. 1 shows the structural formula of [14C]β-d-Glc-IPM, which is labeled in the alkylating moiety. Starting with potassium [14C]cyanide, [14C]ethanolamine hydrochloride was prepared (8) and converted to the chloroethylamine-hydrochloride by reaction with thionylchloride. The phosphoric acid-phenylesterdiamide was synthesized, purified, and the specific activity adjusted to 0.4 GBq/mmol by adding nonradioactive compound (9). Then the phenylester was hydrogenated and coupled to 2,3,4,6-tetrabenzyl-d-glucose-trichloroacetimidate. Purification of the benzylated β-conjugate and its separation from the corresponding α-anomer was achieved by preparative HPLC [Purospher-Si80–5y, 250/25 mm; ethylacetate/n-hexane 90/10 (vol/vol); flow, 10.0 ml × min−1; UV detection at 254 nm]. After hydrogenation and column chromatography on silica gel, highly purified [14C]β-d-Glc-IPM was obtained as checked by HPLC and liquid scintillation counting (Lichrospher-100-APS-5y, 250/4 mm; CH3CN/H2O, 93/7; flow, 1.0 ml; UV detection at 200 nm). The corresponding l-isomer with the same specific activity (0.2–0.6 GBq/mmol) was synthesized with l-glucose following the same procedure. Both 14C-labeled compounds were stored as ethanolic solutions at −20°C and were stable for at least 2 months.
Figure 1.
Chemical structure of [14C]β-d-Glc-IPM. The position of 14C label in the radioactive material is marked by asterisks.
Purospher-Si80 and Lichrospher-100 were obtained from Merck. K14CN (1.9 GBq/mmol), α-methyl-d-[14C]glucopyranoside ([14C]AMG) (11 GBq/mmol), and myo-[23H]inositol (0.7 TBq/mmol) were delivered by Amersham, and [3H]d-glucose (1.3 TBq/mmol) was delivered by Du Pont de Nemours (Dreieich, Germany). SGLT1 (rabbit) (10) was obtained from E. M. Wright, SMIT (dog) (11) was obtained from H. M. Kwon, SGLT1 (man) (12) came from M. J. Coady, Hu14 (man) (13, 14) from M. A. Hediger, and SAAT1 (pig) (6) came from J. E. Lever.
Preparation of RNA, PCRs, and Hybridizations.
Sense cRNAs from rabbit SGLT1 (10), canine SMIT (11), human SGLT1 (12), human Hu14 (13, 14), and porcine SAAT1 (6) were prepared from pBluescript II plasmids (SGLT1s and SMIT) or from pOG vectors (SAAT1, Hu14) as described earlier (15–17). Total RNA was isolated (18) from dissected human renal carcinomas with different grades of malignancy (19), from human tumor xenografts [colon carcinoma CXF 1103; ovary carcinoma OVXF 899 (20)] and from human carcinoma cell lines (kidney carcinoma cell lines KTCTL30 and KTCTL104; colon carcinoma cell lines T84 and HCT-8). The RNAs were treated 10 min at 37°C with DNase (2 units DNase/μg RNA) to remove traces of genomic DNA and subjected to reverse transcriptase–PCR (RT-PCR). RT-PCR was performed with Titan One Tube RT-PCR System (Boehringer Mannheim) following the manufacturer’s recommendation, using 1 μg of RNA and two primers specific for human SAAT1 [accession number U41897 (21)]. The primers were 5′-GGA CTT CCC CAG TAA TGT-3′ (forward, position 93–110) and 5′-CTT CTC GGT AAA GCT CTC-3′ (reverse, position 473–490). PCR products were separated on 1% agarose gel, blotted, and hybridized at 48°C to the 32P-labeled internal oligonucleotide 5′-CAT CTG TGT GGT TTT GTT A-3′ (forward, position 238–256). The membrane was washed at 48°C with 6× SSC containing 0.05% (wt/vol) sodium pyrophosphate. In control experiments with genomic DNA, any cDNA amplification from genomic DNA was excluded.
Expression of Na+ Cotransporters in Xenopus Oocytes and Transport Measurements.
Oocytes were collected, defolliculated, and injected with 50 nl/oocyte of water without or with 2–10 ng of cRNA as described earlier (15). For optimal expression, the oocytes were incubated for either 3 days at 19°C (SGLT1s, SMIT) or 4 days at 15°C plus 2 days at 19°C (SAAT1, Hu14) in 100 mM NaCl/5 mM Hepes-Tris, pH 7.4/3 mM KCl/2 mM CaCl2/1 mM MgCl2 (Ori buffer) containing 50 mg/liter gentamycin. For tracer influx measurements the oocytes were incubated in the absence or presence of phlorizin with Ori buffer (21°C) containing the indicated concentrations of [14C]AMG, [3H]d-glucose, [3H]myo-inositol, [14C]β-d-Glc-IPM, or [14C]β-l-Glc-IPM. In some experiments Na+ in the Ori buffer was partially or totally replaced by tetramethylammonium. After incubation, radioactivity in the oocytes was analyzed (15). The electric properties of β-d-Glc-IPM uptake were investigated by employing the two-microelectrode voltage-clamp technique (17). For the determination of current–voltage relationships, steady-state currents were measured during the last 100 ms of 500-ms rectangular voltage pulses to different potentials. The pulses were applied from a holding potential of −50 mV at a frequency of 0.4 Hz.
Transport Measurements with Human Carcinoma Cells.
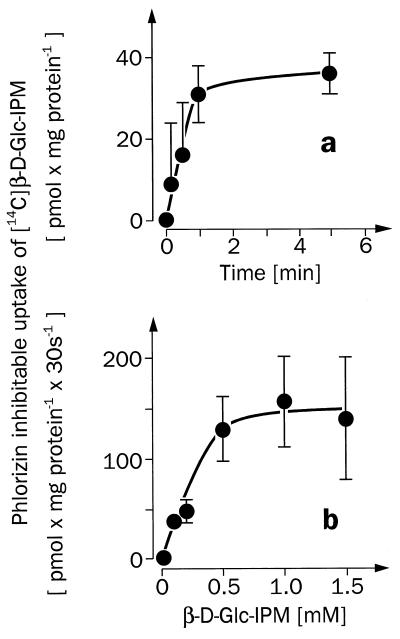
T84 cells (ECACC no. 88021101) provided by European Collection of Animal Cell Culture (Salisbury, U.K.) were grown and subcultured as recommended by the supplier. After confluence the cells were detached by incubation for 1 h at 37°C with Mg2+- and Ca2+-free PBS containing 0.5 mM EDTA, 10 mM Hepes, and 28 mM NaHC03, pH 7.4, sedimented at 1,000 g, and resuspended in PBS. Uptake was measured at 37°C as described earlier (22). In the absence or presence of 0.5 mM phlorizin, the suspended cells were incubated for different time periods with PBS containing 0.2 mM [14C]β-d-Glc-IPM (Fig. 7a), or for 30 s with PBS containing different concentrations of [14C]β-d-Glc-IPM (Fig. 7b). The incubations were stopped with ice-cold PBS containing 1 mM phlorizin. The cells were washed twice with this stop solution, solubilized with 4 M guanidine thiocyanate, and analyzed for radioactivity.
Figure 7.
[14C]β-d-Glc-IPM uptake into suspended human carcinoma T84 cells, which is mediated by a phlorizin-sensitive transporter. (a) The time course of the phlorizin-inhibitable uptake of 0.2 mM [14C]β-d-Glc-IPM measured in the presence of sodium. (b) The substrate dependence of the initial phlorizin-inhibitable uptake rate of [14C]β-d-Glc-IPM, which was measured in the presence of Na+. Mean values ± SEM from four separate determinations are shown. In the presence of 0.5 mM phlorizin, nonspecific uptake of [14C]β-d-Glc-IPM was measured, which increased linearly with time and substrate concentration. The nonspecific uptake rate of [14C]β-d-Glc-IPM was 73 ± 2 pmol × mg −1 mM−1 min−1.
Data Analysis.
The presented tracer flux measurements with Xenopus oocytes represent medians ± SEM from individual measurements in 8–10 oocytes. The demonstrated electrical measurements with oocytes and the tracer uptake experiments with colon carcinoma cells are mean values ± SEM from three or four individual determinations, respectively. The Michaelis–Menten equation was fitted to the data in Figs. 4, 5, and 7b, and the apparent Km values ± SD were calculated.
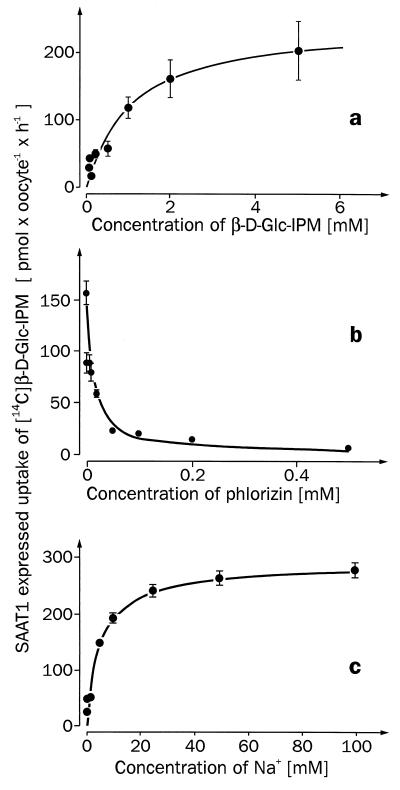
Figure 4.
Substrate dependence (a), phlorizin inhibition (b), and Na+ dependence (c) of the expressed [14C]β-d-Glc-IPM uptake by SAAT1. Xenopus oocytes were injected with 50 nl of water without or with 10 ng of SAAT1-cRNA and incubated for 5 days. In both types of oocytes initial uptake rates of [14C]β-d-Glc-IPM were measured after 30 min of incubation, and the expressed uptake rates were calculated. (a) The expressed [14C]β-d-Glc-IPM uptake measured in Ori buffer containing different concentrations of [14C]β-d-Glc-IPM. (b) The expressed uptake of 0.8 mM [14C]β-d-Glc-IPM is shown, which was measured in Ori buffer containing different concentrations of phlorizin. (c) The expressed uptake rate of 0.8 mM [14C]β-d-Glc-IPM measured in the presence of different sodium concentrations. Here sodium in the Ori buffer was replaced by tetramethylammonium.
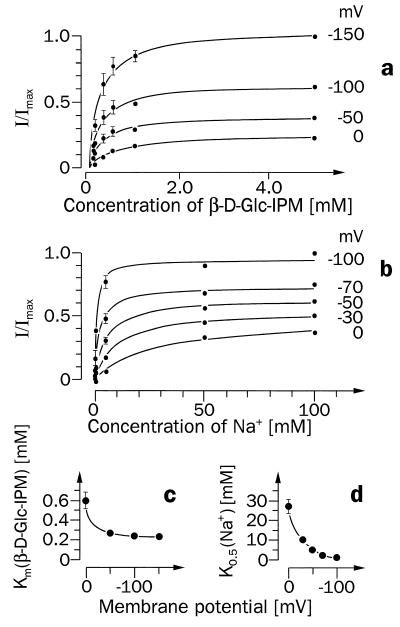
Figure 5.
Potential dependence of SAAT1-mediated currents induced by different concentrations of β-d-Glc-IPM in the presence of Na+ (a and c) or by different Na+ concentrations in the presence of β-d-Glc-IPM (b and d). Ten nanograms of SAAT1-cRNA was injected into Xenopus oocytes, and the oocytes were incubated for 5 days. (a) The currents are shown that were measured when oocytes clamped at different membrane potentials were superfused with different concentrations of β-d-Glc-IPM in the presence of 100 mM Na+. The measurements from three oocytes were normalized to their maximal currents at −150 mV. The Michaelis–Menten equation was fitted to the data obtained at the respective membrane potential, and the Km values were plotted against the different membrane potentials (c). The currents measured after superfusion of the oocytes with 1 mM β-d-Glc-IPM in the presence of different concentrations of Na+ are shown in b. Here measurements from three oocytes were normalized to their current in the presence of 100 mM Na+ at −100 mV. Means and SEM values are presented, and the Michaelis–Menten equation was fitted to the currents obtained at each membrane potential. The K0.5 values for Na+ activation at different membrane potentials are presented in d.
RESULTS
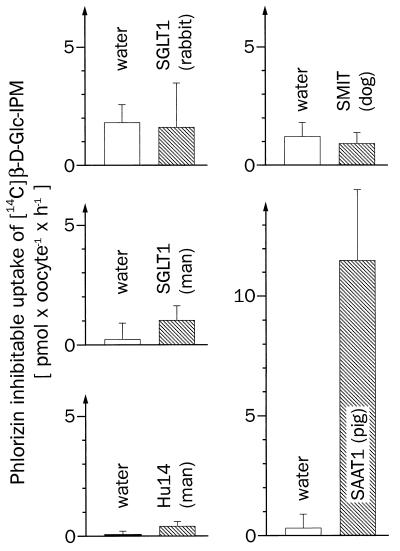
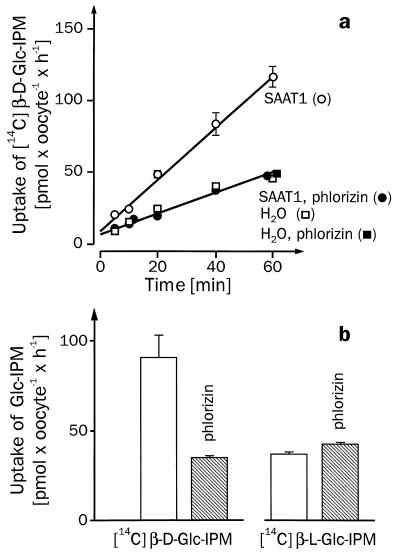
Previous data suggested that the cytotoxic effect of β-d-Glc-IPM was reduced by phlorizin (4). Therefore, we investigated whether β-d-Glc-IPM is transported by one of the phlorizin-sensitive Na+-d-glucose cotransporters that belong to the transporter family of SGLT1 (5, 10). We expressed most of the cloned SGLT1-type Na+-sugar cotransporters in oocytes of Xenopus laevis and determined whether they mediated phlorizin-inhibitable uptake of [14C]β-d-Glc-IPM. The following transporters were studied: the high-affinity Na+-d-glucose cotransporter SGLT1 from rabbit (10), the high-affinity Na+-d-glucose cotransporter SGLT1 from man (12), the low-affinity Na+-d-glucose cotransporter Hu14 from man (13, 14), the low-affinity Na+-d-glucose cotransporter SAAT1 from pig (6, 7), and the Na+-myo-inositol cotransporter SMIT from dog (11). In the experiments, the function of the expressed transporters was always controlled by measuring the expression of phlorizin-inhibitable uptake of [14C]AMG (SGLT1s, SAAT1, Hu14) or of [3H]myo-inositol (SMIT). Fig. 2 shows that significant phlorizin-inhibitable transport of [14C]β-d-Glc-IPM was only observed with SAAT1. These experiments have been performed at least three times. β-d-Glc-IPM uptake expressed by SAAT1 was linear for 1 h (Fig. 3a). Fig. 3b shows that the uptake was stereospecific. A linear uptake of [14C]β-d-Glc-IPM was also observed into the control oocytes injected with water; however, this uptake could not be inhibited by phlorizin (Fig. 3a) and was not stereospecific (data not shown). The functional properties of β-d-Glc-IPM uptake by SAAT1 were further characterized in oocytes. Fig. 4a shows the substrate dependence of the expressed [14C]β-d-Glc-IPM uptake. Substrate saturation was observed, and an apparent Km value of 1.1 ± 0.3 mM was estimated. For the uptake of [14C]AMG and [3H]d-glucose by SAAT1, we obtained Km values of 1.9 ± 0.3 mM and 2.7 ± 0.4 mM, respectively. In one batch of oocytes, Vmax values of 0.19 ± 0.02 (β-d-Glc-IPM), 1.2 ± 0.1 (AMG), and 0.8 ± 0.1 (d-glucose) nmol × oocyte−1 × h−1 were determined. In Fig. 4b the concentration dependence for the phlorizin inhibition of [14C]β-d-Glc-IPM uptake was measured. A Ki value of 4.4 ± 1.0 μM was estimated. It was similar to the Ki value of 10 ± 2 μM, which we determined for phlorizin inhibition of AMG uptake. Fig. 4c shows the sodium dependence of [14C]β-d-Glc-IPM uptake by SAAT1 employing a β-d-Glc-IPM concentration of 0.8 mM. Half-maximal activation was observed at 4.4 ± 0.7 mM.
Figure 2.
Na+-sugar cotransporters homologous to SGLT1 were tested for their capability to transport [14C]β-d-Glc-IPM. Xenopus laevis oocytes were injected with 50 nl of water with or without 10 ng cRNA of SGLT1 (rabbit), SGLT1 (man), Hu14 (man), SMIT (dog), or SAAT1 (pig). After 3–6 days of incubation, the expression of the respective transporter was controlled by measuring the phlorizin-inhibitable uptake after 30 min of incubation with 50 μM [14C]AMG (SGLT1s, SAAT1), 1.25 mM [14C]AMG (Hu14), and 1 μM [3H]myo-inositol (SMIT). The employed phlorizin concentrations were 100 μM (SGLT1 from rabbit), 200 μM (SGLT1 from man, Hu14, SAAT1), or 500 μM (SMIT). The expressed phlorizin-inhibitable uptake rates of AMG and myo-inositol were 274 ± 22 (SGLT1, rabbit), 48 ± 5 (SGLT1, man), 25 ± 1 (Hu14), 6.5 ± 0.7 (SMIT), and 18 ± 2 (SAAT1) pmol × oocyte−1 × h−1, respectively. Under identical experimental conditions the same batches of injected oocytes were tested for phlorizin-inhibitable uptake of 100 μM [14C]β-d-Glc-IPM. Medians and SEM values from 8–10 parallel determinations without and with phlorizin are indicated.
Figure 3.
Time course (a) and stereospecificity (b) of Glc-IPM uptake expressed by SAAT1. Xenopus oocytes were injected with 50 nl of water without (squares in a) or with 10 ng of SAAT1-cRNA (circles in a, and all experiments in b) and incubated for 6 days. Uptake of 200 μM [14C]β-d-Glc-IPM or [14C]β-l-Glc-IPM was measured after incubating the oocytes for different time periods (a) or for 30 min (b) either in Ori buffer or in Ori buffer containing 175 μM phlorizin. Uptake rates of [14C]β-d-Glc-IPM and [14C]β-l-Glc-IPM in SAAT1-cRNA-injected oocytes are shown in b. In oocytes injected with water identical uptake rates for [14C]β-d-Glc-IPM and [14C]β-l-Glc-IPM were measured. These rates were insensitive to phlorizin and not significantly different from those in oocytes injected with SAAT1-cRNA in the presence of phlorizin (data not shown). Medians from 8–10 oocytes and SEM are indicated. Typical experiments out of three are presented.
Electrical measurements were performed for a more detailed analysis. Oocytes expressing SAAT1 were superfused with different concentrations of β-d-Glc-IPM in the presence of 100 mM Na+, and the induced inward currents were measured at different membrane potentials (Fig. 5a and c). The maximal induced currents at saturating β-d-Glc-IPM concentrations increased with increasing membrane potential (Fig. 5a). Over a wide range of membrane potentials (−50 to −150 mV), a relatively constant Km value for β-d-Glc-IPM of 0.25 ± 0.02 mM was determined (Fig. 5c). However, when the membrane potential was clamped to zero the Km value for β-d-Glc-IPM increased to 0.61 ± 0.08 mM, which is similar to the Km value determined by tracer flux. This suggests that during the tracer flux measurements the membranes of the oocytes were depolarized. For transport of d-glucose, a Km value of 6.0 mM has been reported in oocytes expressing SAAT1, which were clamped to −150 mV (7, 23). The Km for AMG was constant over a wide range of membrane potentials and increased only slightly at 0 mV (7, 23). For the substrate dependence of β-d-Glc-IPM uptake by SAAT1 at different membrane potentials, we calculated Hill coefficients around 1 (0.95–1.1), as has been reported for AMG (7). The voltage dependence of the sodium activation of β-d-Glc-IPM uptake by SAAT1 is shown in Fig. 5 b and d. The results are similar to those obtained for AMG uptake (7, 23). The concentration of sodium inducing a half-maximal current in the presence of 1 mM β-d-Glc-IPM decreased with increasing membrane potential (from 27 ± 8 mM at 0 mV to 0.8 ± 0.4 mM at −100 mV). In the voltage range between −30 and −100 mV, Hill coefficients around 0.7 (0.69–0.74) were estimated. These data are consistent with a 1:1 stoichiometry for Na+ and β-d-Glc-IPM, as has been proposed for cotransport of Na+ and AMG (7). The currents induced after superfusion with saturating substrate concentrations (10 mM) were obtained in the same oocyte batch that was used to determine the Vmax values by tracer flux (see above). With these oocytes clamped at −50 mV, currents of −13 ± 1 nA (β-d-Glc-IPM, n = 4), −132 ± 20 nA (d-glucose, n = 4), and −147 ± 26 nA (AMG, n = 4) were determined. In the range of −120 to −20 mV a similar voltage dependence was obtained for the maximal currents induced by the three substrates (data not shown). Taken together, our data suggest that SAAT1 translocates glucose and β-d-Glc-IPM by the same mechanism. At physiologically relevant membrane potentials, β-d-Glc-IPM is transported with an ≈10-fold higher affinity and 10 times smaller maximal velocity than d-glucose or AMG.
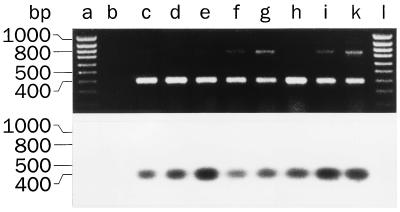
To evaluate whether SAAT1 may transport β-d-Glc-IPM into human tumor cells, we investigated whether SAAT1 was transcribed in human tumors and tumor cell lines and measured phlorizin-inhibitable β-d-Glc-IPM uptake into one of the cell lines. Apparently, SAAT1 is also expressed in man because we cloned a 438-bp cDNA fragment from human brain that had a 86% nucleotide identity to SAAT1 and encoded 83% identical amino acids (6, 21). Employing PCRs with reverse-transcribed RNAs we showed that this human SAAT1 fragment is transcribed in carcinomas from kidney, colon, and ovary, in colon carcinoma T84 cells, and in two renal carcinoma cell lines (Fig. 6). No transcription of SAAT1 was detected in the human cell line HCT-8, which has been derived from an ileocecal adenocarcinoma (data not shown). We investigated whether a phlorizin-inhibitable β-d-Glc-IPM uptake system is functional in T84 cells. Fig. 7 shows uptake experiments with [14C]β-d-Glc-IPM. Although in the presence of phlorizin a large nonspecific uptake of [14C]β-d-Glc-IPM was observed (see legend of Fig. 7), phlorizin-inhibitable uptake of [14C]β-d-Glc-IPM could be demonstrated. This phlorizin-inhibitable uptake of [14C]β-d-Glc-IPM was linear for about 1 min and showed substrate saturation with an apparent Km value of 0.39 ± 0.18 mM. The data suggest that the uptake of β-d-Glc-IPM into human colon carcinoma cells is mediated by the Na+-d-glucose cotransporter SAAT1.
Figure 6.
Transcription of SAAT1 in human tumor cells. Total RNA was isolated from differentially graded human renal carcinomas (lane c, nuclear grade-1 tumor; lane d, nuclear grade-2 tumor; lane e, nuclear grade-3 tumor), from mouse tumor xenografts (lane f, human colon carcinoma; lane g, human ovary carcinoma), and from the human carcinoma cell lines KTCTL30 (lane h), KTCTL104 (lane i), or T84 (lane k). The RNAs and a control without RNA (lane b) were reverse transcribed, and PCRs were performed with primers that were specific for the cloned fragment of the human SAAT1. The amplification products, size markers, and the control were separated on an agarose gel and stained with ethidium bromide (Upper) or hybridized with an internal primer specific for the human SAAT1 (Lower).
DISCUSSION
This paper identifies a secondary active sodium cotransporter that translocates the alkylating agent β-d-Glc-IPM across the plasma membrane. This is an important step in defining the prospective specificity and toxicity of this new alkylating cytostatic, which has been developed from ifosfamide (24–27) and is currently being tested in clinical studies. Both the classical cytostatic cyclophosphamide and ifosfamide are prodrugs that are hydroxylated in the liver by cytochrome P450 and isomerize to open-ring aldose forms that decompose to acrolein and the alkylating metabolites phosphoramide mustard or isophosphoramide mustard, respectively (27). The release of acrolein may lead to hemorrhagic cystitis (26). In the conjugate β-d-Glc-IPM the isophosphoramide mustard is stabilized by coupling to d-glucose via a β-glucoside linkage, whereby the isophosphoramide mustard became more water soluble. The intriguing observation of the present paper that β-d-Glc-IPM is transported by the low-affinity Na+-d-glucose cotransporter SAAT1 indicates that intact β-d-Glc-IPM is transported into cells where it may be activated by hydrolysis, either spontaneously or catalyzed by intracellular glucosidases (28). More generally, our data suggest that some of the organic solute transporters in the plasma membrane may be used for drug targeting.
Because the complete SAAT1 cDNA has only been cloned from pig (7, 11), our functional analysis was performed with this transporter. Transport of β-d-Glc-IPM by SAAT1 showed functional properties that were very similar to the transport of d-glucose or AMG. This indicates that SAAT1 translocates β-d-Glc-IPM by the same mechanism as glucose and may accumulate β-d-Glc-IPM in the cells, as has been reported for d-glucose (5). Our data show that [14C]-l-Glc-IPM is not transported by SAAT1. This indicates that in the tracer uptake experiments shown above, [14C]β-d-Glc-IPM is transported rather than the [14C]isophosphoramide mustard, which might have been formed by hydrolysis and may be responsible for the relatively high, nonspecific uptake observed in our experiments. At physiologically relevant membrane potentials between −50 and −100 mV, the affinity for β-d-Glc-IPM of pig SAAT1 is 10 times higher than for d-glucose, whereas the turnover of β-d-Glc-IPM is about 10 times lower. It has been reported earlier that some β-phenylglucosides interact with the high-affinity Na+-d-glucose cotransporter SGLT1 from rabbit (29). In contrast to noninteracting α-phenylglucosides, the β-phenylglucosides may be competitive inhibitors of glucose transport by SGLT1 like phlorizin and p-nitrophenylglucoside, or may be transported by SGLT1 like arbutin (4-[hydroxy]phenyl-β-d-glucopyranoside), helicin (salicil-aldehyde-β-d-glucopyranoside), and cycasin (methylazoxymethanol-β-d-glucoside) (29, 30). However, for transport of β-glucosides by SGLT1, rather limited structural requirements must be fulfilled, and substantially lower affinities were estimated for the transported β-glucosides than for d-glucose.
Our data strongly suggest that a SAAT1-type Na+-d-glucose cotransporter that translocates β-d-Glc-IPM is also expressed in the human. In man, we identified a cDNA fragment encoding 163 aa with 83% identity to pig SAAT1 (21). β-d-Glc-IPM is probably also transported by the analogous human SAAT1-transporter. We measured β-d-Glc-IPM uptake into human carcinoma cells where this gene is transcribed. The uptake is phlorizin-inhibitable and has an apparent Km of 0.4 mM. It was an intriguing observation that this analogous human SAAT1 gene is transcribed in various human tumors and tumor cell lines, because this could mean that tumor cells are especially sensitive to alkylation by β-d-Glc-IPM. To determine the clinical significance of β-d-Glc-IPM transport by SAAT1 in human tumor cells, future work is required. First, the human SAAT1 transporter must be cloned and β-d-Glc-IPM transport characterized. In addition, the expression, functional activity, and regulation of SAAT1 in different human tissues and tumor cells have to be determined. The pharmacokinetics of β-d-Glc-IPM in human and the hydrolysis of β-d-Glc-IPM in human tumor cells are under study. In rat, β-d-Glc-IPM disperses within minutes between blood and intracellular space and it is mainly excreted in the kidney. After an intravenous bolus administration of β-d-Glc-IPM, about 75% of the administered β-d-Glc-IPM is excreted into the urine after 8 h, one-fifth of which is hydrolyzed (31). Also, in man the interstitial concentration of β-d-Glc-IPM may rapidly drop after a bolus injection. In this situation the concentrative uptake of β-d-Glc-IPM into tumor cells mediated by SAAT1 may help to reach therapeutically relevant concentrations. This may be the reason for the large cytostatic activity of β-d-Glc-IPM that has been observed in clinical phase 1 studies. After short time infusions with β-d-Glc-IPM, progressive tumor growing was stopped in several patients, and in one patient with renal cancer a significant regression of the tumor mass was observed (personal communication from J. Pohl, Asta Medica).
Acknowledgments
We thank E. M. Wright, H. M. Known, M. J. Coady, and M. A. Hediger for supplying us with SGLT-type transporters, and W. Schwarz for providing us with software for the analysis of voltage-clamp measurements. Human tumors were supplied by H. W. Birk and H. H. Fiebig, and M. Christof prepared the figures. This work was supported by Sonderforschungsbereich 176, Grant A17.
ABBREVIATIONS
- β-d-Glc-IPM
β-d-glucosylisophosphoramide mustard
- AMG
methyl-α-d-glucopyranoside
References
- 1.Schwartsmann G, Workman P. Eur J Cancer. 1993;29A:3–14. doi: 10.1016/0959-8049(93)90567-y. [DOI] [PubMed] [Google Scholar]
- 2.Tietze, L. F., Fischer, R., Beller, M. & Seele, R. (1990) Liebigs Ann. Chem. 151–157.
- 3.Schwabe K, Graffi A, Redslob J, Hülsmann W, Butschak G. Pharmazie. 1976;31:438–441. [PubMed] [Google Scholar]
- 4.Pohl J, Bertram B, Hilgard P, Nowrousian M R, Stüben J, Wießler M. Cancer Chemother Pharmacol. 1995;35:364–370. doi: 10.1007/s002800050248. [DOI] [PubMed] [Google Scholar]
- 5.Hediger M A, Rhoads D B. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 6.Kong C-T, Yet S-F, Lever J E. J Biol Chem. 1993;268:1509–1512. [PubMed] [Google Scholar]
- 7.Mackenzie B, Panayotova-Heiermann M, Loo D D F, Lever J E, Wright E M. J Biol Chem. 1994;269:22488–22491. [PubMed] [Google Scholar]
- 8.Koltai E, Horvath B, Banfi D. J Lab Comp Radiopharm. 1982;19:7–11. [Google Scholar]
- 9.Lorenz P, Wiessler M. Arch Pharm. 1985;318:577–582. [Google Scholar]
- 10.Hediger M A, Coady M J, Ikeda T S, Wright E M. Nature (London) 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 11.Kwon H M, Yamauchi A, Uchida S, Preston A S, Garcia-Perez A, Burg M B, Handler J S. J Biol Chem. 1992;267:6297–6301. [PubMed] [Google Scholar]
- 12.Hediger M A, Turk E, Wright E M. Proc Natl Acad Sci USA. 1989;86:5748–5752. doi: 10.1073/pnas.86.15.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells R G, Pajor A M, Kanai Y, Turk E, Wright E M, Hediger M A. Am J Physiol. 1992;263:F459–F465. doi: 10.1152/ajprenal.1992.263.3.F459. [DOI] [PubMed] [Google Scholar]
- 14.Kanai Y, Lee W-S, You G, Brown D, Hediger M A. J Clin Invest. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veyhl M, Spangenberg J, Püschel B, Poppe R, Dekel C, Fritzsch G, Haase W, Koepsell H. J Biol Chem. 1993;268:25041–25053. [PubMed] [Google Scholar]
- 16.Lambotte S, Veyhl M, Köhler M, Morrison-Shetlar A I, Kinne R K H, Schmid M, Koepsell H. DNA Cell Biol. 1996;15:769–777. doi: 10.1089/dna.1996.15.769. [DOI] [PubMed] [Google Scholar]
- 17.Busch A E, Quester S, Ulzheimer J C, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. J Biol Chem. 1996;271:32599–32604. doi: 10.1074/jbc.271.51.32599. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrman S A, Lasky L C, Limas C. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Fiebig H H, Berger D P, Dengler W A, Wallbrecher E, Winterhalter B R. In: Combined in Vitro/in Vivo Test Procedure with Human Tumor Xenografts for New Drug Development. Fiebig H H, Berger D P, editors. Vol. 42. 1992. pp. 321–351. [Google Scholar]
- 21.Poppe R, Karbach U, Gambaryan S, Wiesinger H, Lutzenburg M, Kraemer M, Witte O W, Koepsell H. J Neurochem. 1997;69:84–94. doi: 10.1046/j.1471-4159.1997.69010084.x. [DOI] [PubMed] [Google Scholar]
- 22.Busch A E, Quester S, Ulzheimer J C, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. FEBS Lett. 1996;395:153–156. doi: 10.1016/0014-5793(96)01030-7. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie B, Loo D D F, Panayotova-Heiermann M, Wright E M. J Biol Chem. 1996;271:32678–32683. doi: 10.1074/jbc.271.51.32678. [DOI] [PubMed] [Google Scholar]
- 24.Arnold H, Bourseaux F, Brock N. Arzneim-Forsch. 1961;11:143–158. [PubMed] [Google Scholar]
- 25.Brock N. Arzneim-forsch. 1958;8:1–9. [PubMed] [Google Scholar]
- 26.Brock N. Cancer Res. 1989;49:1–7. [PubMed] [Google Scholar]
- 27.Lind M J, Ardiet C. Cancer Surv. 1993;17:157–188. [PubMed] [Google Scholar]
- 28.Gopalan V, Pastuszyn A, Galey W R, Jr, Glew R H. J Biol Chem. 1992;267:14027–14032. [PubMed] [Google Scholar]
- 29.Lostao M P, Hirayama B A, Loo D D F, Wright E M. J Membr Biol. 1994;142:161–170. doi: 10.1007/BF00234938. [DOI] [PubMed] [Google Scholar]
- 30.Hirayama B, Hazama A, Loo D F, Wright E M, Kisby G E. Biochim Biophys Acta. 1994;1193:151–154. doi: 10.1016/0005-2736(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 31.Stüben J, Port R, Bertram B, Bollow U, Hull W E, Schaper M, Pohl J, Wiessler M. Cancer Chemother Pharmacol. 1996;38:355–365. doi: 10.1007/s002800050495. [DOI] [PubMed] [Google Scholar]