Abstract
The ORF6 gene product of Mycoplasma pneumoniae is involved in a yet-unknown manner in the adhesion of the bacterium to its host cell. Part of the ORF6 gene is a repetitive DNA sequence (RepMP5), about 1,900 bp long. Seven additional similar copies of RepMP5 are dispersed on the genome. In the independently isolated strains M. pneumoniae M129 and FH, the RepMP5 copies residing in the ORF6 gene are not identical. Two conserved regions, ranging from nucleotides 1 to 799 and from nucleotide 1795 to the end of the gene, border a variable region, ranging from nucleotides 800 to 1794. This variable region differs in DNA sequence and by 201 bp. Analysis of RepMP5 copies outside the ORF6 gene showed that both M. pneumoniae M129 and M. pneumoniae FH carry a RepMP5 copy on a 6-kbp EcoRI fragment which has the same DNA sequence as the variable region of RepMP5 in the M. pneumoniae FH ORF6 gene. According to these data, a switch from the M. pneumoniae M129 ORF6 gene to the M. pneumoniae FH ORF6 gene could take place by gene conversion.
Full text
PDF

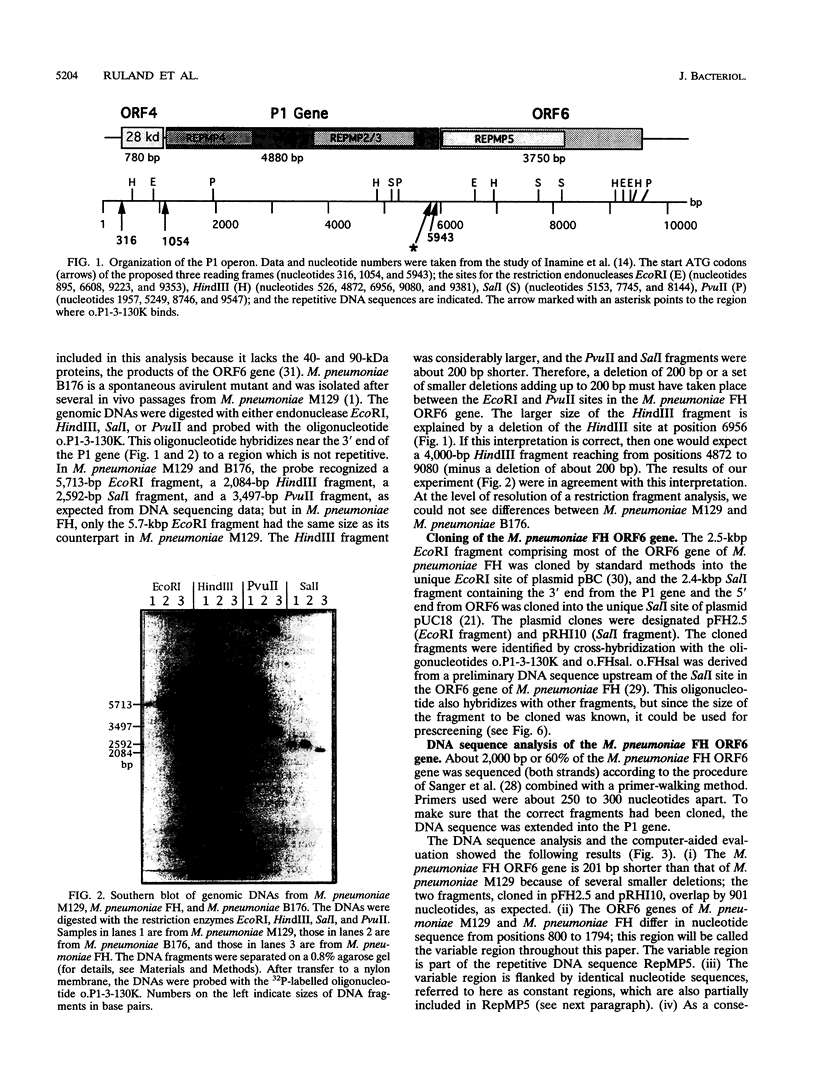





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988 Sep;56(9):2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Bott K. F. Prevalence of novel repeat sequences in and around the P1 operon in the genome of Mycoplasma pneumoniae. Gene. 1990 Mar 1;87(1):91–96. doi: 10.1016/0378-1119(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Baseman J. B. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect Immun. 1990 Dec;58(12):4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Woodard A. Cloning and DNA sequence of a mycoplasmal recA gene. J Bacteriol. 1992 Feb;174(3):778–784. doi: 10.1128/jb.174.3.778-784.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Ferretti L., Karnik S. S., Khorana H. G., Nassal M., Oprian D. D. Total synthesis of a gene for bovine rhodopsin. Proc Natl Acad Sci U S A. 1986 Feb;83(3):599–603. doi: 10.1073/pnas.83.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G., Hu P. C., Meloni G. A., Barile M. F. The immunodominant 90-kilodalton protein is localized on the terminal tip structure of Mycoplasma pneumoniae. Infect Immun. 1993 Apr;61(4):1523–1530. doi: 10.1128/iai.61.4.1523-1530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary S. J., Gabridge M. G., Intres R., Draper D. L., Gladd M. F. Identification of Mycoplasma binding proteins utilizing a 100 kilodalton lung fibroblast receptor. J Recept Res. 1989;9(6):465–478. doi: 10.3109/10799898909066071. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Loechel S., Hu P. C. Analysis of the nucleotide sequence of the P1 operon of Mycoplasma pneumoniae. Gene. 1988 Dec 15;73(1):175–183. doi: 10.1016/0378-1119(88)90323-x. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Layh-Schmitt G., Herrmann R. Localization and biochemical characterization of the ORF6 gene product of the Mycoplasma pneumoniae P1 operon. Infect Immun. 1992 Jul;60(7):2906–2913. doi: 10.1128/iai.60.7.2906-2913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Childs R. A., Paulson J. C., Rogers G. N., Scudder P. R., Michalski J. C., Hounsell E. F., Taylor-Robinson D., Feizi T. Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type. Nature. 1984 Feb 9;307(5951):560–563. doi: 10.1038/307560a0. [DOI] [PubMed] [Google Scholar]
- Loveless R. W., Feizi T. Sialo-oligosaccharide receptors for Mycoplasma pneumoniae and related oligosaccharides of poly-N-acetyllactosamine series are polarized at the cilia and apical-microvillar domains of the ciliated cells in human bronchial epithelium. Infect Immun. 1989 Apr;57(4):1285–1289. doi: 10.1128/iai.57.4.1285-1289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Razin S., Jacobs E. Mycoplasma adhesion. J Gen Microbiol. 1992 Mar;138(3):407–422. doi: 10.1099/00221287-138-3-407. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Olson L. D., Barile M. F., Ginsburg V., Krivan H. C. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J Biol Chem. 1989 Jun 5;264(16):9289–9293. [PubMed] [Google Scholar]
- Robertson B. D., Meyer T. F. Genetic variation in pathogenic bacteria. Trends Genet. 1992 Dec;8(12):422–427. doi: 10.1016/0168-9525(92)90325-x. [DOI] [PubMed] [Google Scholar]
- Ruland K., Wenzel R., Herrmann R. Analysis of three different repeated DNA elements present in the P1 operon of Mycoplasma pneumoniae: size, number and distribution on the genome. Nucleic Acids Res. 1990 Nov 11;18(21):6311–6317. doi: 10.1093/nar/18.21.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperker B., Hu P., Herrmann R. Identification of gene products of the P1 operon of Mycoplasma pneumoniae. Mol Microbiol. 1991 Feb;5(2):299–306. doi: 10.1111/j.1365-2958.1991.tb02110.x. [DOI] [PubMed] [Google Scholar]
- Su C. J., Chavoya A., Baseman J. B. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988 Dec;56(12):3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Chavoya A., Dallo S. F., Baseman J. B. Sequence divergency of the cytadhesin gene of Mycoplasma pneumoniae. Infect Immun. 1990 Aug;58(8):2669–2674. doi: 10.1128/iai.58.8.2669-2674.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Dallo S. F., Chavoya A., Baseman J. B. Possible origin of sequence divergence in the P1 cytadhesin gene of Mycoplasma pneumoniae. Infect Immun. 1993 Mar;61(3):816–822. doi: 10.1128/iai.61.3.816-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Physical mapping of the Mycoplasma pneumoniae genome. Nucleic Acids Res. 1988 Sep 12;16(17):8323–8336. doi: 10.1093/nar/16.17.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Repetitive DNA sequences in Mycoplasma pneumoniae. Nucleic Acids Res. 1988 Sep 12;16(17):8337–8350. doi: 10.1093/nar/16.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]






