Abstract
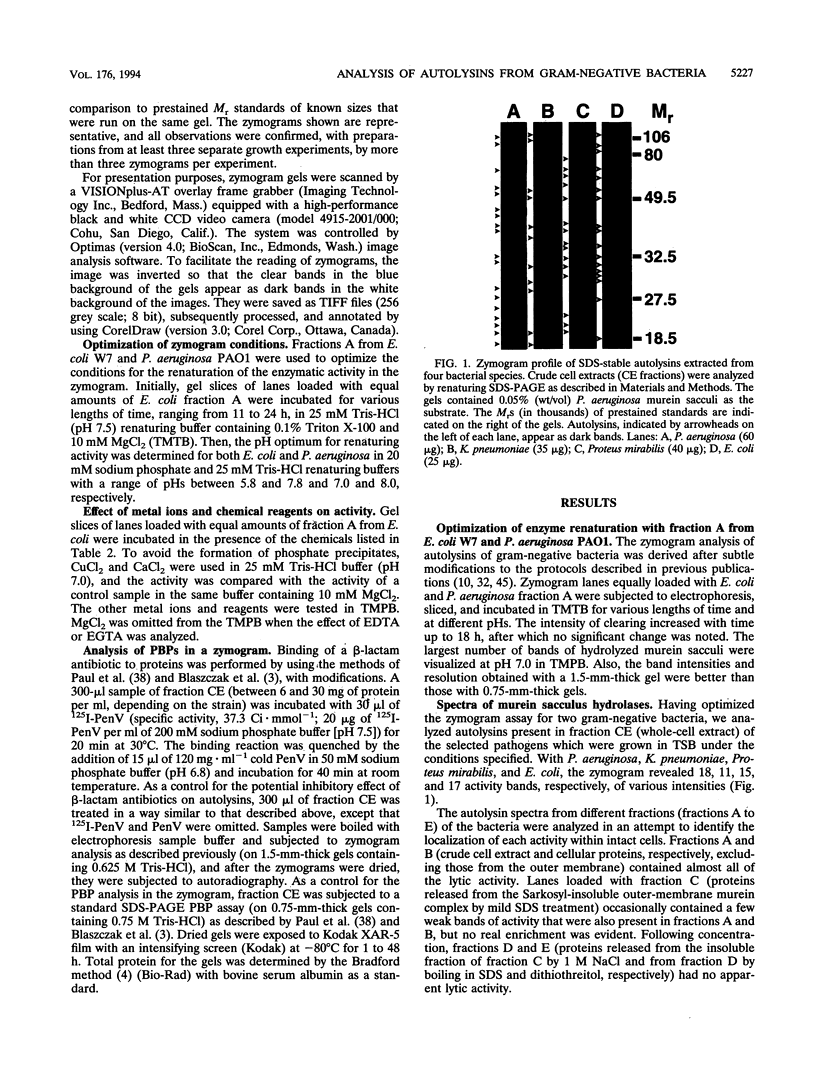
For the first time, peptidoglycan autolysins from cellular fractions derived from sonicated cultures of Pseudomonas aeruginosa PAO1, Escherichia coli W7, Klebsiella pneumoniae CWK2, and Proteus mirabilis 19 were detected and partially characterized by zymogram analysis. Purified murein sacculi from P. aeruginosa PAO1 were incorporated into a sodium dodecyl sulfate (SDS)-polyacrylamide gel at a concentration of 0.05% (wt/vol) to serve as a substrate for the separated autolysins. At least 11 autolysin bands of various intensities with M(r)s ranging between 17,000 and 122,000 were detected in each of the homogenated cultures. Some of the autolysins of the four bacteria had similar M(r)s. The zymogram analysis was used to show that a number of the autolysins from E. coli were inhibited by the heavy metals Hg2+ and Cu2+, at 1 and 10 mM, respectively, high ionic strengths, and reagents known to affect the packing of lipopolysaccharides. The activity of an autolysin with an M(r) of 65,000 was also impaired by penicillin G, whereas it was enhanced by gentamicin. A preliminary screen to determine the relationship between penicillin-binding proteins (PBPs) and autolysins was carried out by using a dual assay in which radiolabelled penicillin V bands were visualized on an autolysin zymogram. Radiolabelled bands corresponding to PBPs 3, 4, 5, and 6 from E. coli and P. aeruginosa; PBPs 3, 4, and 6 from Proteus mirabilis; and PBP 6 from K. pneumoniae degraded the murein sacculi in the gels and were presumed to have autolytic activity, although the possibility of two distinct enzymes, each with one of the activities, comigrating in the SDS-polyacrylamide gels could not be excluded. Some radiolabelled bands possessed an Mr of <34,000 and coincided with similar low-Mr autolysin bands.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck B. D., Park J. T. Activity of three murein hydrolases during the cell division cycle of Escherichia coli K-12 as measured in toluene-treated cells. J Bacteriol. 1976 Jun;126(3):1250–1260. doi: 10.1128/jb.126.3.1250-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. M., Lock R. A., Hansman D., Paton J. C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989 Aug;57(8):2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brito N., Falcón M. A., Carnicero A., Gutiérrez-Navarro A. M., Mansito T. B. Purification and peptidase activity of a bacteriolytic extracellular enzyme from Pseudomonas aeruginosa. Res Microbiol. 1989 Feb;140(2):125–137. doi: 10.1016/0923-2508(89)90046-6. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Ioannidis I., Edelman A., Spratt B. G. Nucleotide sequences of the penicillin-binding protein 5 and 6 genes of Escherichia coli. Nucleic Acids Res. 1988 Feb 25;16(4):1617–1617. doi: 10.1093/nar/16.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Daneo-Moore L., Shockman G. D. The second peptidoglycan hydrolase of Streptococcus faecium ATCC 9790 covalently binds penicillin. J Bacteriol. 1989 Aug;171(8):4355–4361. doi: 10.1128/jb.171.8.4355-4361.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster S. J. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992 Jan;174(2):464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J., Negayama K., Takigawa K., Yamagishi Y., Yamaji Y., Kawanishi K., Takahara J. In-vitro activity of imipenem and amikacin combinations against resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 1993 Jun;31(6):1007–1009. doi: 10.1093/jac/31.6.1007. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E., Rabin H. R. beta-Lactam-resistant Pseudomonas aeruginosa with modified penicillin-binding proteins emerging during cystic fibrosis treatment. Antimicrob Agents Chemother. 1981 May;19(5):705–711. doi: 10.1128/aac.19.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Henderson T. A., Dombrosky P. M., Young K. D. Artifactual processing of penicillin-binding proteins 7 and 1b by the OmpT protease of Escherichia coli. J Bacteriol. 1994 Jan;176(1):256–259. doi: 10.1128/jb.176.1.256-259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle B. D., Beveridge T. J. Metal binding by the peptidoglycan sacculus of Escherichia coli K-12. Can J Microbiol. 1984 Feb;30(2):204–211. doi: 10.1139/m84-031. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tuomanen E. I. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J Gen Microbiol. 1991 Mar;137(3):441–454. doi: 10.1099/00221287-137-3-441. [DOI] [PubMed] [Google Scholar]
- Iida K., Hirota Y., Schwarz U. Mutants of Escherichia coli defective in penicillin-insensitive murein DD-endopeptidase. Mol Gen Genet. 1983;189(2):215–221. doi: 10.1007/BF00337807. [DOI] [PubMed] [Google Scholar]
- Jayaswal R. K., Lee Y. I., Wilkinson B. J. Cloning and expression of a Staphylococcus aureus gene encoding a peptidoglycan hydrolase activity. J Bacteriol. 1990 Oct;172(10):5783–5788. doi: 10.1128/jb.172.10.5783-5788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Clarke A. J., Beveridge T. J. Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol. 1993 Sep;175(18):5798–5805. doi: 10.1128/jb.175.18.5798-5805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Lam J. S., Beveridge T. J. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob Agents Chemother. 1993 Apr;37(4):715–721. doi: 10.1128/aac.37.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck W., Wientjes F. B., Schwarz U. Comparison of two hydrolytic murein transglycosylases of Escherichia coli. Eur J Biochem. 1985 May 2;148(3):493–497. doi: 10.1111/j.1432-1033.1985.tb08866.x. [DOI] [PubMed] [Google Scholar]
- Keck W., van Leeuwen A. M., Huber M., Goodell E. W. Cloning and characterization of mepA, the structural gene of the penicillin-insensitive murein endopeptidase from Escherichia coli. Mol Microbiol. 1990 Feb;4(2):209–219. doi: 10.1111/j.1365-2958.1990.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Additional arguments for the key role of "smart" autolysins in the enlargement of the wall of gram-negative bacteria. Res Microbiol. 1990 Jun;141(5):529–541. doi: 10.1016/0923-2508(90)90017-k. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc D., Asselin A. Detection of bacterial cell wall hydrolases after denaturing polyacrylamide gel electrophoresis. Can J Microbiol. 1989 Aug;35(8):749–753. doi: 10.1139/m89-125. [DOI] [PubMed] [Google Scholar]
- Maller R., Ahrne H., Holmen C., Lausen I., Nilsson L. E., Smedjegård J. Once- versus twice-daily amikacin regimen: efficacy and safety in systemic gram-negative infections. Scandinavian Amikacin Once Daily Study Group. J Antimicrob Chemother. 1993 Jun;31(6):939–948. doi: 10.1093/jac/31.6.939. [DOI] [PubMed] [Google Scholar]
- Martin N. L., Beveridge T. J. Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob Agents Chemother. 1986 Jun;29(6):1079–1087. doi: 10.1128/aac.29.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett H., Keck W., Funk A., Schwarz U. Two different species of murein transglycosylase in Escherichia coli. J Bacteriol. 1980 Oct;144(1):45–52. doi: 10.1128/jb.144.1.45-52.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Ohya S., Yamazaki M., Sugawara S., Matsuhashi M. Penicillin-binding proteins in Proteus species. J Bacteriol. 1979 Jan;137(1):474–479. doi: 10.1128/jb.137.1.474-479.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul T. R., Halligan N. G., Blaszczak L. C., Parr T. R., Jr, Beveridge T. J. A new mercury-penicillin V derivative as a probe for ultrastructural localization of penicillin-binding proteins in Escherichia coli. J Bacteriol. 1992 Jul;174(14):4689–4700. doi: 10.1128/jb.174.14.4689-4700.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peetz R. H., Kenny G. E. Prevention of autolysis in suspensions of Neisseria gonorrhoeae by mercuric ions. J Bacteriol. 1978 Jul;135(1):283–285. doi: 10.1128/jb.135.1.283-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J. M., Jackson M. E., Holland I. B. The C terminus of penicillin-binding protein 5 is essential for localisation to the E. coli inner membrane. EMBO J. 1986 Sep;5(9):2399–2405. doi: 10.1002/j.1460-2075.1986.tb04510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Sugai M., Akiyama T., Komatsuzawa H., Miyake Y., Suginaka H. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureus bacteriolytic enzymes by polyacrylamide gel electrophoresis. J Bacteriol. 1990 Nov;172(11):6494–6498. doi: 10.1128/jb.172.11.6494-6498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó I., Penyige A., Barabás G., Barabás J. Effect of aminoglycoside antibiotics on the autolytic enzyme of Streptomyces griseus. Arch Microbiol. 1990;155(1):99–102. doi: 10.1007/BF00291282. [DOI] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Tipper D. J. Mode of action of beta-lactam antibiotics. Pharmacol Ther. 1985;27(1):1–35. doi: 10.1016/0163-7258(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomioka S., Matsuhashi M. Purification of penicillin-insensitive DD-endopeptidase, a new cell wall peptidoglycan-hydrolyzing enzyme in Escherichia coli, and its inhibition by deoxyribonucleic acids. Biochem Biophys Res Commun. 1978 Oct 30;84(4):978–984. doi: 10.1016/0006-291x(78)91679-0. [DOI] [PubMed] [Google Scholar]
- Van Heijenoort Y., Van Heijenoort J. Study of the N-acetylmuramyl-L-alanine amidase activity in Escherichia coli. FEBS Lett. 1971 Jun 10;15(2):137–141. doi: 10.1016/0014-5793(71)80041-8. [DOI] [PubMed] [Google Scholar]
- Walderich B., Höltje J. V. Subcellular distribution of the soluble lytic transglycosylase in Escherichia coli. J Bacteriol. 1991 Sep;173(18):5668–5676. doi: 10.1128/jb.173.18.5668-5676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- van Heijenoort J., Parquet C., Flouret B., van Heijenoort Y. Envelope-bound N-acetylmuramyl-L-alanine amidase of Escherichia coli K 12. Purification and properties of the enzyme. Eur J Biochem. 1975 Oct 15;58(2):611–619. doi: 10.1111/j.1432-1033.1975.tb02412.x. [DOI] [PubMed] [Google Scholar]





