Abstract
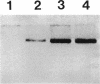
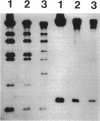
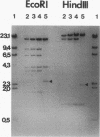
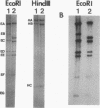
A self-transmissible 2,4-dichlorophenoxyacetic acid (2,4-D)-degradative plasmid, pKA2, has been identified in a new 2,4-D-degrading strain, Alcaligenes paradoxus 2811P, isolated from agricultural soil. pKA2 occurred as a 42.9-kb plasmid in strain 2811P. A derivative strain, 2811C, was isolated from a stock culture in which the entire pKA2 plasmid was apparently integrated into the host chromosome without loss of the 2,4-D+ phenotype. This interpretation is based on the disappearance of a free plasmid DNA band, a shift in the tfdA-hybridizing band to the chromosome, loss of transmissibility of the 2,4-D+ trait, and appropriate shifts in Southern hybridization bands of plasmid DNA compared with whole-cell DNA. The integrated plasmid of strain 2811C was excised either precisely or imprecisely after continued transfer on 2,4-D-containing medium. This suggests that a chromosome-free plasmid cycle may occur to optimize fitness under conditions of specific resource fluctuation. Another new 2,4-D-degrading strain, Pseudomonas pickettii 712, which was isolated from the same field plot but at a different time, was found to carry a plasmid that is nearly identical to pKA2. The plasmid of this strain, pKA4, is 40.9 kb long and has features in common with pKA2, such as high self-transmissibility, hybridization only to the tfdA gene among the 2,4-D-metabolic genes of 2,4-D-degradative plasmid pJP4, and similar restriction endonuclease-generated fragments. Furthermore, the genetic homology between the two plasmids was high since all fragments of pKA2 hybridized to pKA4. These results suggest that these two plasmids are closely related and thus their occurrence in two genera in nature is the result of natural horizontal gene transfer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carney B. F., Leary J. V. Novel Alterations in Plasmid DNA Associated with Aromatic Hydrocarbon Utilization by Pseudomonas putida R5-3. Appl Environ Microbiol. 1989 Jun;55(6):1523–1530. doi: 10.1128/aem.55.6.1523-1530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981 Feb;145(2):681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. R., Appleton J., Pemberton J. M. Isolation and characterization of the pesticide-degrading plasmid pJP1 from Alcaligenes paradoxus. J Bacteriol. 1978 Sep;135(3):798–804. doi: 10.1128/jb.135.3.798-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Meyer M., Schlegel H. G. Transfer and expression of the herbicide-degrading plasmid pJP4 in aerobic autotrophic bacteria. Arch Microbiol. 1983 Feb;134(2):92–97. doi: 10.1007/BF00407938. [DOI] [PubMed] [Google Scholar]
- Fulthorpe R. R., Wyndham R. C. Survival and activity of a 3-chlorobenzoate-catabolic genotype in a natural system. Appl Environ Microbiol. 1989 Jun;55(6):1584–1590. doi: 10.1128/aem.55.6.1584-1590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. M., Brookes D. E., Stokes H. W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991 Aug;5(8):1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Holben W. E., Schroeter B. M., Calabrese V. G., Olsen R. H., Kukor J. K., Biederbeck V. O., Smith A. E., Tiedje J. M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992 Dec;58(12):3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeenes D. J., Williams P. A. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J Bacteriol. 1982 Apr;150(1):188–194. doi: 10.1128/jb.150.1.188-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka J. O., Holben W. E., Tiedje J. M. Analysis of competition in soil among 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl Environ Microbiol. 1994 Apr;60(4):1121–1128. doi: 10.1128/aem.60.4.1121-1128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka J. O., Holben W. E., Tiedje J. M. Genetic and phenotypic diversity of 2,4-dichlorophenoxyacetic acid (2,4-D)-degrading bacteria isolated from 2,4-D-treated field soils. Appl Environ Microbiol. 1994 Apr;60(4):1106–1115. doi: 10.1128/aem.60.4.1106-1115.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritz T., Ernst A., Black T. A., Wolk C. P. High-resolution mapping of genetic loci of Anabaena PCC 7120 required for photosynthesis and nitrogen fixation. Mol Microbiol. 1993 Apr;8(1):101–110. doi: 10.1111/j.1365-2958.1993.tb01207.x. [DOI] [PubMed] [Google Scholar]
- Meulien P., Broda P. Identification of chromosomally integrated TOL DNA in cured derivatives of Pseudomonas putida PAW1. J Bacteriol. 1982 Nov;152(2):911–914. doi: 10.1128/jb.152.2.911-914.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Fisher P. R. 2,4-D plasmids and persistence. Nature. 1977 Aug 25;268(5622):732–733. doi: 10.1038/268732a0. [DOI] [PubMed] [Google Scholar]
- Regensburger A., Ludwig W., Schleifer K. H. DNA probes with different specificities from a cloned 23S rRNA gene of Micrococcus luteus. J Gen Microbiol. 1988 May;134(5):1197–1204. doi: 10.1099/00221287-134-5-1197. [DOI] [PubMed] [Google Scholar]
- Sinclair M. I., Maxwell P. C., Lyon B. R., Holloway B. W. Chromosomal location of TOL plasmid DNA in Pseudomonas putida. J Bacteriol. 1986 Dec;168(3):1302–1308. doi: 10.1128/jb.168.3.1302-1308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wyndham R. C., Singh R. K., Straus N. A. Catabolic instability, plasmid gene deletion and recombination in Alcaligenes sp. BR60. Arch Microbiol. 1988;150(3):237–243. doi: 10.1007/BF00407786. [DOI] [PubMed] [Google Scholar]