Abstract
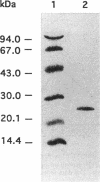
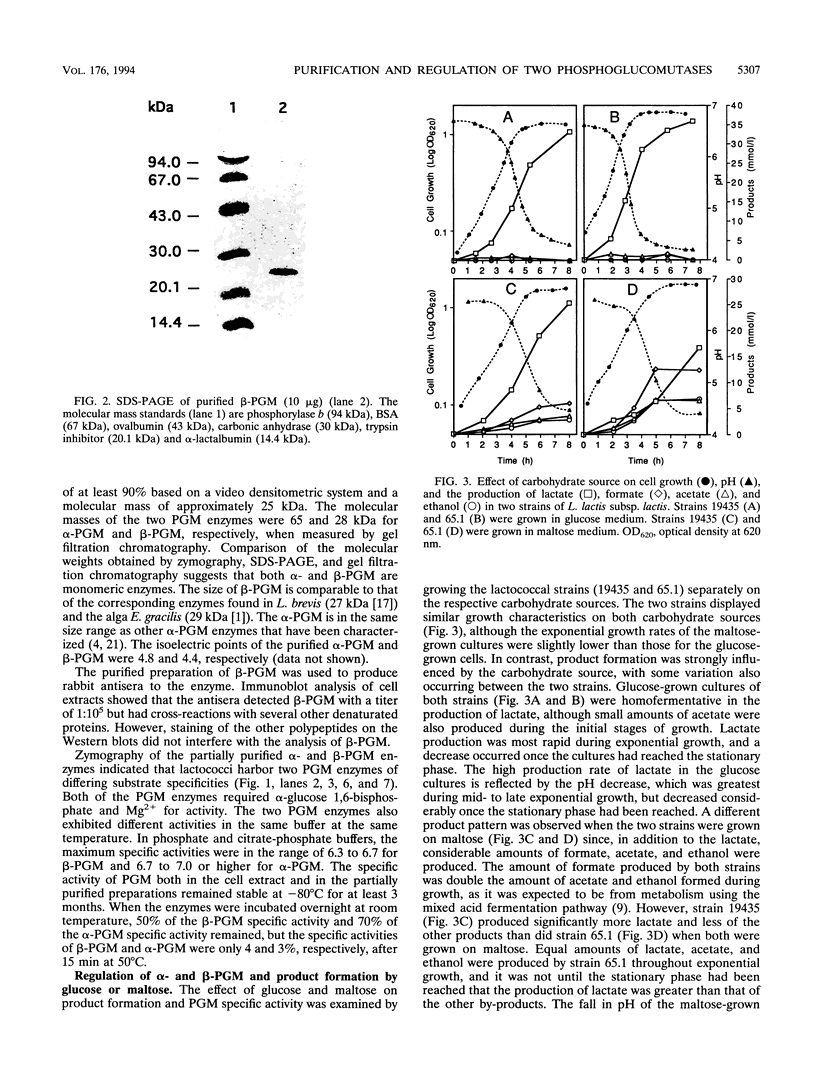
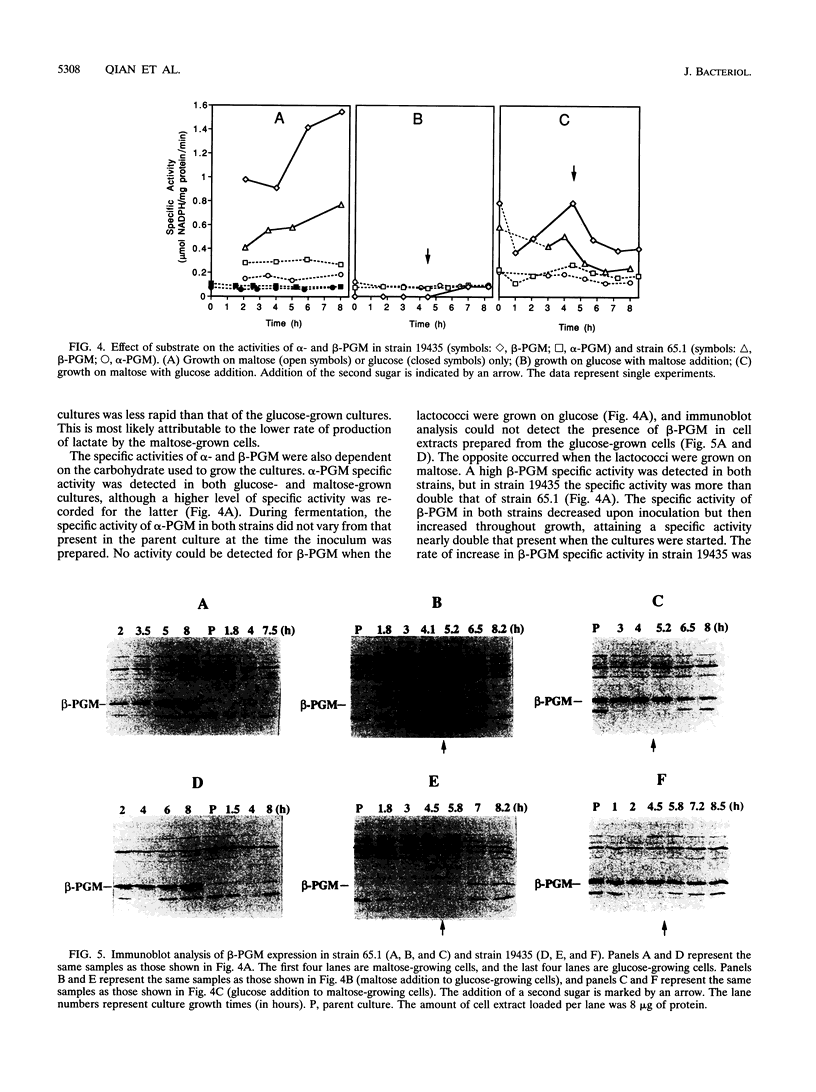
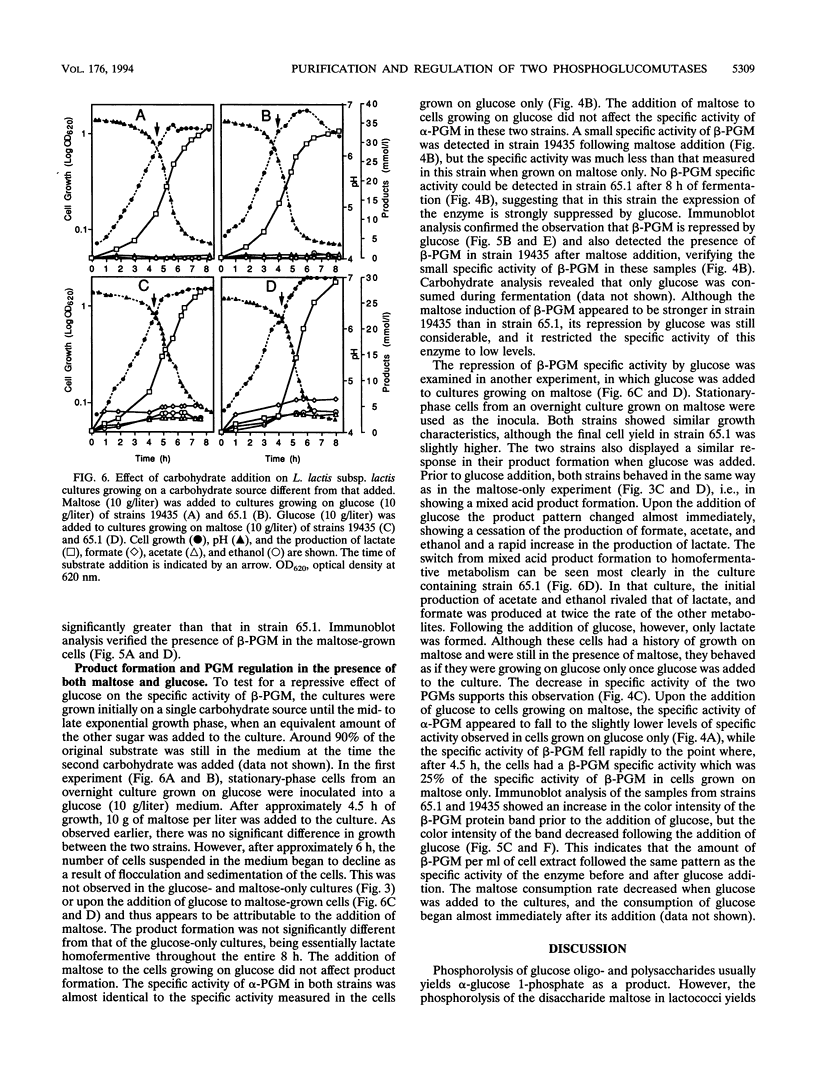
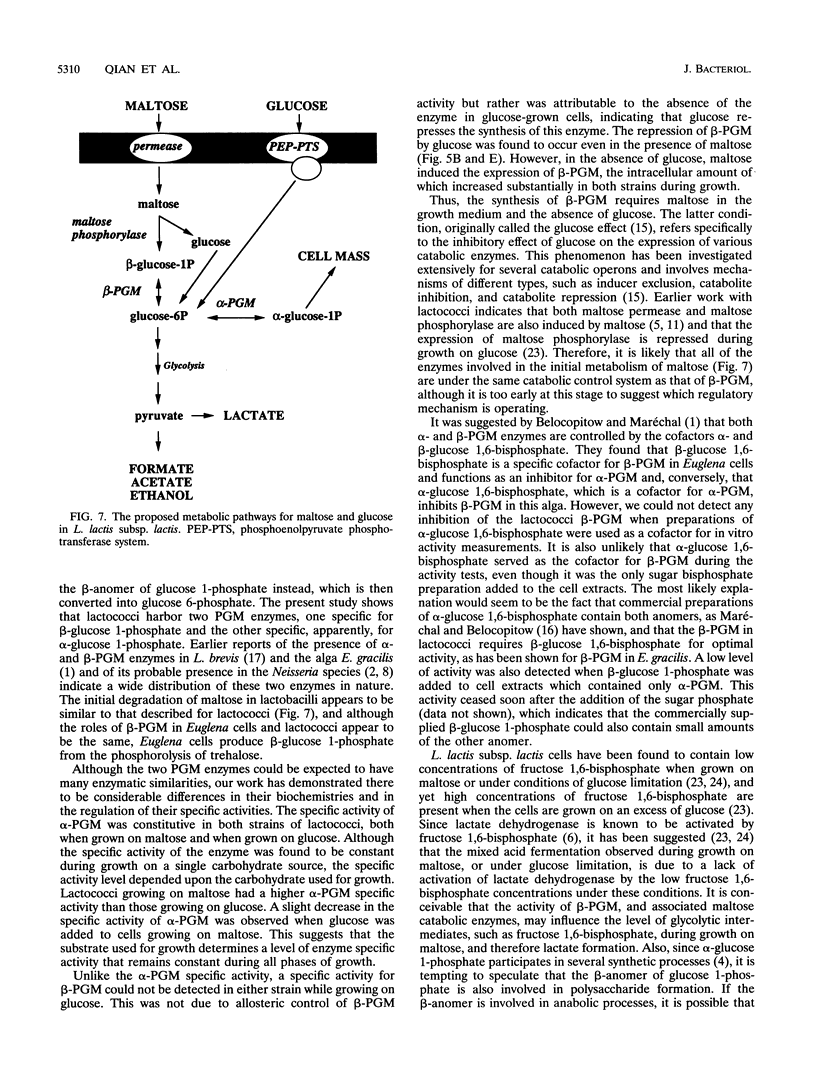
Two distinct forms of phosphoglucomutase were found in Lactococcus lactis subsp. lactis, strains 19435 and 65.1, growing on maltose: beta-phosphoglucomutase (beta-PGM), which catalyzes the reversible conversion of beta-glucose 1-phosphate to glucose 6-phosphate in the maltose catabolism, and alpha-phosphoglucomutase (alpha-PGM). beta-PGM was purified to more than 90% homogeneity in crude cell extract from maltose-grown lactococci, and polyclonal antisera to the enzyme were prepared. The molecular mass of beta-PGM was estimated by gel filtration to be 28 kDa; its isoelectric point was 4.8. The corresponding values for alpha-PGM were 65 kDa and 4.4, respectively. The expression of both PGM enzymes was investigated under different growth conditions. The specific activity and amount of beta-PGM per milliliter of cell extract increased with time in lactococci grown on maltose, but the enzyme was absent in lactococci grown on glucose, indicating enzyme synthesis to be induced by maltose in the growth medium. When glucose was added to maltose-grown lactococci, both the specific activity and amount of beta-PGM per milliliter of cell extract decreased rapidly. This suggests that synthesis of beta-PGM is repressed by glucose in the medium. Although the specific activity of alpha-PGM did not change during growth on maltose or glucose, lactococcal strain 19435 showed a much higher specific activity of both alpha- and beta-PGM than strain 65.1 when grown on maltose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belocopitow E., Maréchal L. R. Metabolism of trehalose in Euglena gracilis. Partial purification and some properties of phosphoglucomutase acting on beta-glucose 1-phosphate. Eur J Biochem. 1974 Aug 1;46(3):631–637. doi: 10.1111/j.1432-1033.1974.tb03659.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brautaset T., Standal R., Fjaervik E., Valla S. Nucleotide sequence and expression analysis of the Acetobacter xylinum phosphoglucomutase gene. Microbiology. 1994 May;140(Pt 5):1183–1188. doi: 10.1099/13500872-140-5-1183. [DOI] [PubMed] [Google Scholar]
- Citti J. E., Sandine W. E., Elliker P. R. Lactose and maltose uptake by Streptococcus lactis. J Dairy Sci. 1967 Apr;50(4):485–487. doi: 10.3168/jds.S0022-0302(67)87451-4. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Fructose 1,6-diphosphate-activated L-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J Bacteriol. 1977 Jul;131(1):82–91. doi: 10.1128/jb.131.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Peist R., Reidl J., Kossmann M., Brand B., Boos W. Maltose and maltotriose can be formed endogenously in Escherichia coli from glucose and glucose-1-phosphate independently of enzymes of the maltose system. J Bacteriol. 1993 Sep;175(17):5655–5665. doi: 10.1128/jb.175.17.5655-5665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITTING C., DOUDOROFF M. Phosphorolysis of maltose by enzyme preparations from Neisseria meningitidis. J Biol Chem. 1952 Nov;199(1):153–163. [PubMed] [Google Scholar]
- Fordyce A. M., Crow V. L., Thomas T. D. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl Environ Microbiol. 1984 Aug;48(2):332–337. doi: 10.1128/aem.48.2.332-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSHI J. G., HANDLER P. PHOSPHOGLUCOMUTASE. I. PURIFICATION AND PROPERTIES OF PHOSPHOGLUCOMUTASE FROM ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:2741–2751. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marechal L. R., Oliver G., Veiga L. A., de Ruiz Holgado A. A. Partial purification and some properties of beta-phosphoglucomutase from Lactobacillus brevis. Arch Biochem Biophys. 1984 Feb 1;228(2):592–599. doi: 10.1016/0003-9861(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal L. R., Belocopitow E. Metabolism of trehalose in Euglena gracilis. Beta glucose 1,6-bisphosphate, activation factor of phosphoglucomutase for beta glucose 1-phosphate. Eur J Biochem. 1974 Feb 15;42(1):45–50. doi: 10.1111/j.1432-1033.1974.tb03312.x. [DOI] [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Role of galactose or glucose-1-phosphate in preventing the lysis of Streptococcus diacetilactis. J Bacteriol. 1968 Feb;95(2):592–602. doi: 10.1128/jb.95.2.592-602.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W., Jacobson G. R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993 Sep;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg A., Hahn-Hägerdal B. beta-Glucose-1-Phosphate, a Possible Mediator for Polysaccharide Formation in Maltose-Assimilating Lactococcus lactis. Appl Environ Microbiol. 1989 Jun;55(6):1549–1554. doi: 10.1128/aem.55.6.1549-1554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]