Abstract
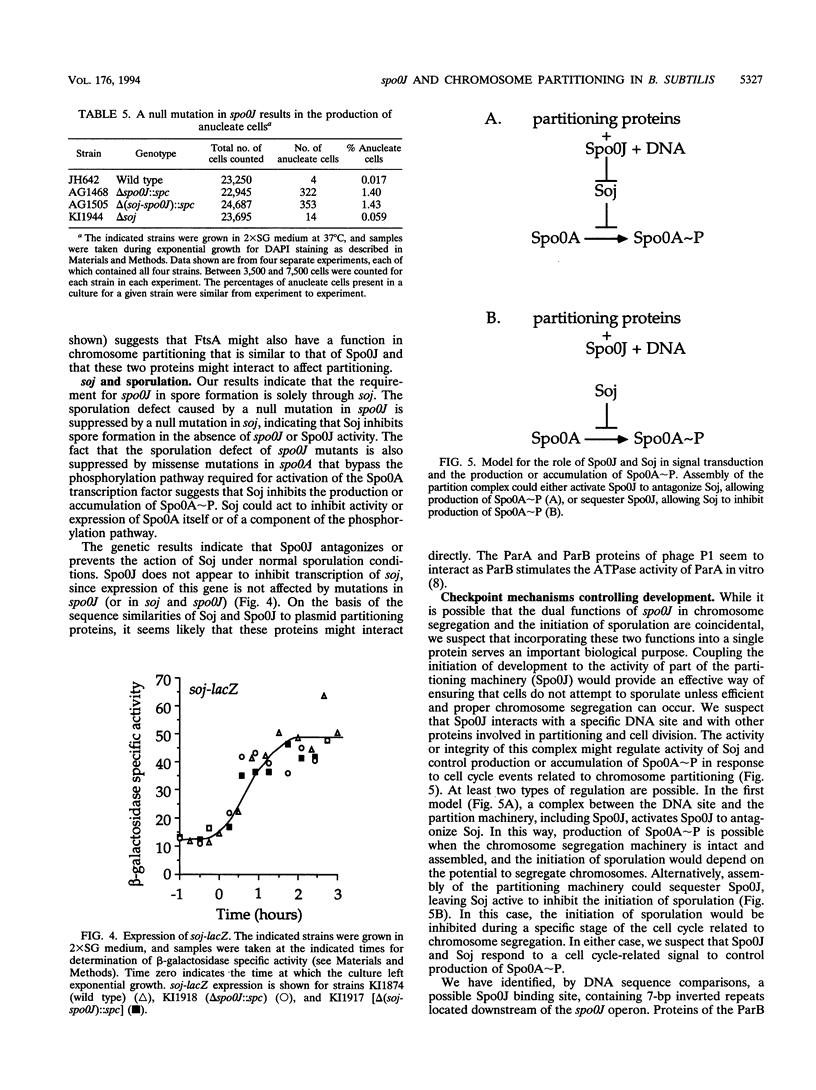
The spo0J gene of Bacillus subtilis is required for the initiation of sporulation. We show that the sporulation defect caused by null mutations in spo0J is suppressed by a null mutation in the gene located directly upstream from spo0J, soj (suppressor of spo0J). These results indicate that Soj inhibits the initiation of sporulation and that Spo0J antagonizes that inhibition. Further genetic experiments indicated that Soj ultimately affects sporulation by inhibiting the activation (phosphorylation) of the developmental transcription factor encoded by spo0A. In addition, the temperature-sensitive sporulation phenotype caused by the ftsA279 (spoIIN279) mutation was partly suppressed by the soj null mutation, indicating that FtsA might also affect the activity of Soj. Soj and Spo0J are known to be similar in sequence to a family of proteins involved in plasmid partitioning, including ParA and ParB of prophage P1, SopA and SopB of F, and IncC and KorB of RK2, spo0J was found to be required for normal chromosome partitioning as well as for sporulation. spo0J null mutants produced a significant proportion of anucleate cells during vegetative growth. The dual functions of Spo0J could provide a mechanism for regulating the initiation of sporulation in response to activity of the chromosome partition machinery.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund T., Bernander R., Nordström K. Cell division in Escherichia coli minB mutants. Mol Microbiol. 1992 Aug;6(15):2073–2083. doi: 10.1111/j.1365-2958.1992.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Antoniewski C., Savelli B., Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J Bacteriol. 1990 Jan;172(1):86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus J. M., Green B. D., Youngman P., Moran C. P., Jr Phosphorylation of Bacillus subtilis transcription factor Spo0A stimulates transcription from the spoIIG promoter by enhancing binding to weak 0A boxes. J Bacteriol. 1994 Jan;176(2):296–306. doi: 10.1128/jb.176.2.296-306.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. K., Haldenwang W. G. Regulation of sigma B levels and activity in Bacillus subtilis. J Bacteriol. 1993 Apr;175(8):2347–2356. doi: 10.1128/jb.175.8.2347-2356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. H., Grimsley J. K., Hoch J. A., Spiegelman G. B. Phosphorylation of Spo0A activates its stimulation of in vitro transcription from the Bacillus subtilis spoIIG operon. Mol Microbiol. 1993 Aug;9(4):741–749. doi: 10.1111/j.1365-2958.1993.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Burbulys D., Trach K. A., Hoch J. A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991 Feb 8;64(3):545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Martin K. A., Austin S. J. Biochemical activities of the parA partition protein of the P1 plasmid. Mol Microbiol. 1992 May;6(9):1141–1147. doi: 10.1111/j.1365-2958.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Lewis T., Levin N., DeVivo R. Suppressors of a spo0A missense mutation and their effects on sporulation in Bacillus subtilis. Biochimie. 1992 Jul-Aug;74(7-8):679–688. doi: 10.1016/0300-9084(92)90140-a. [DOI] [PubMed] [Google Scholar]
- Guzmán P., Westpheling J., Youngman P. Characterization of the promoter region of the Bacillus subtilis spoIIE operon. J Bacteriol. 1988 Apr;170(4):1598–1609. doi: 10.1128/jb.170.4.1598-1609.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes F., Radnedge L., Davis M. A., Austin S. J. The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol Microbiol. 1994 Jan;11(2):249–260. doi: 10.1111/j.1365-2958.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Hiraga S. Chromosome and plasmid partition in Escherichia coli. Annu Rev Biochem. 1992;61:283–306. doi: 10.1146/annurev.bi.61.070192.001435. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Trach K., Kawamura F., Saito H. Identification of the transcriptional suppressor sof-1 as an alteration in the spo0A protein. J Bacteriol. 1985 Feb;161(2):552–555. doi: 10.1128/jb.161.2.552-555.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Grossman A. D. A developmental checkpoint couples the initiation of sporulation to DNA replication in Bacillus subtilis. EMBO J. 1994 Apr 1;13(7):1566–1573. doi: 10.1002/j.1460-2075.1994.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Grossman A. D. Coupling between gene expression and DNA synthesis early during development in Bacillus subtilis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8808–8812. doi: 10.1073/pnas.89.18.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Grossman A. D. Interactions among mutations that cause altered timing of gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1992 May;174(10):3185–3195. doi: 10.1128/jb.174.10.3185-3195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Rudner D. Z., Siranosian K. J., Grossman A. D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993 Feb;7(2):283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Hiraga S. Minicell-forming mutants of Escherichia coli: production of minicells and anucleate rods. J Bacteriol. 1988 Jul;170(7):3094–3101. doi: 10.1128/jb.170.7.3094-3101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmazyn-Campelli C., Fluss L., Leighton T., Stragier P. The spoIIN279(ts) mutation affects the FtsA protein of Bacillus subtilis. Biochimie. 1992 Jul-Aug;74(7-8):689–694. doi: 10.1016/0300-9084(92)90141-z. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Saito H. Isolation and mapping of a new suppressor mutation of an early sporulation gene spoOF mutation in Bacillus subtilis. Mol Gen Genet. 1983;192(3):330–334. doi: 10.1007/BF00392171. [DOI] [PubMed] [Google Scholar]
- Koonin E. V. A superfamily of ATPases with diverse functions containing either classical or deviant ATP-binding motif. J Mol Biol. 1993 Feb 20;229(4):1165–1174. doi: 10.1006/jmbi.1993.1115. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee A., Malak M., Louie P., Arjomand J., Ginther C., Leighton T. Intergenic suppression of stage II sporulation defects by a mutation in the major vegetative sigma factor gene (rpoD) of Bacillus subtilis. Biochimie. 1992 Jul-Aug;74(7-8):635–640. doi: 10.1016/0300-9084(92)90135-2. [DOI] [PubMed] [Google Scholar]
- Leighton T. New types of RNA polymerase mutations causing temperature-sensitive sporulation in bacillus subtilis. J Biol Chem. 1977 Jan 10;252(1):268–272. [PubMed] [Google Scholar]
- Losick R., Stragier P. Crisscross regulation of cell-type-specific gene expression during development in B. subtilis. Nature. 1992 Feb 13;355(6361):601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- Motallebi-Veshareh M., Rouch D. A., Thomas C. M. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol. 1990 Sep;4(9):1455–1463. doi: 10.1111/j.1365-2958.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- Mulder E., El'Bouhali M., Pas E., Woldringh C. L. The Escherichia coli minB mutation resembles gyrB in defective nucleoid segregation and decreased negative supercoiling of plasmids. Mol Gen Genet. 1990 Mar;221(1):87–93. doi: 10.1007/BF00280372. [DOI] [PubMed] [Google Scholar]
- Mysliwiec T. H., Errington J., Vaidya A. B., Bramucci M. G. The Bacillus subtilis spo0J gene: evidence for involvement in catabolite repression of sporulation. J Bacteriol. 1991 Mar;173(6):1911–1919. doi: 10.1128/jb.173.6.1911-1919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H., Jaffé A., Imamura R., Ogura T., Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991 Jan;10(1):183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Yoshikawa H. Genes and their organization in the replication origin region of the bacterial chromosome. Mol Microbiol. 1992 Mar;6(5):629–634. doi: 10.1111/j.1365-2958.1992.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Ohlsen K. L., Grimsley J. K., Hoch J. A. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1756–1760. doi: 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Cole S. P., Burbulys D., Trach K., Hoch J. A. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Hoch J. A. Isolation and sequence of the spo0E gene: its role in initiation of sporulation in Bacillus subtilis. Mol Microbiol. 1987 Jul;1(1):125–132. doi: 10.1111/j.1365-2958.1987.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Perego M., Hoch J. A. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. J Bacteriol. 1991 Apr;173(8):2514–2520. doi: 10.1128/jb.173.8.2514-2520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Spiegelman G. B., Hoch J. A. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol. 1988 Nov;2(6):689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla J., Dopazo A., Vicente M. The native form of FtsA, a septal protein of Escherichia coli, is located in the cytoplasmic membrane. J Bacteriol. 1990 Sep;172(9):5097–5102. doi: 10.1128/jb.172.9.5097-5102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman K., Losick R., Youngman P. Genetic analysis of Bacillus subtilis spo mutations generated by Tn917-mediated insertional mutagenesis. Genetics. 1987 Dec;117(4):603–617. doi: 10.1093/genetics/117.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satola S. W., Baldus J. M., Moran C. P., Jr Binding of Spo0A stimulates spoIIG promoter activity in Bacillus subtilis. J Bacteriol. 1992 Mar;174(5):1448–1453. doi: 10.1128/jb.174.5.1448-1453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satola S., Kirchman P. A., Moran C. P., Jr Spo0A binds to a promoter used by sigma A RNA polymerase during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4533–4537. doi: 10.1073/pnas.88.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Magill N., Febbroriello P., Nakhimovsky L., Koppel D. E., Setlow P. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J Bacteriol. 1991 Oct;173(19):6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji K., Hiratsuka S., Kawamura F., Kobayashi Y. New suppressor mutation sur0B of spo0B and spo0F mutations in Bacillus subtilis. J Gen Microbiol. 1988 Dec;134(12):3249–3257. doi: 10.1099/00221287-134-12-3249. [DOI] [PubMed] [Google Scholar]
- Spiegelman G., Van Hoy B., Perego M., Day J., Trach K., Hoch J. A. Structural alterations in the Bacillus subtilis Spo0A regulatory protein which suppress mutations at several spo0 loci. J Bacteriol. 1990 Sep;172(9):5011–5019. doi: 10.1128/jb.172.9.5011-5019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trach K. A., Hoch J. A. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol Microbiol. 1993 Apr;8(1):69–79. doi: 10.1111/j.1365-2958.1993.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Trach K., Burbulys D., Strauch M., Wu J. J., Dhillon N., Jonas R., Hanstein C., Kallio P., Perego M., Bird T. Control of the initiation of sporulation in Bacillus subtilis by a phosphorelay. Res Microbiol. 1991 Sep-Oct;142(7-8):815–823. doi: 10.1016/0923-2508(91)90060-n. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Howard M. G., Piggot P. J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989 Feb;171(2):692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York K., Kenney T. J., Satola S., Moran C. P., Jr, Poth H., Youngman P. Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE. J Bacteriol. 1992 Apr;174(8):2648–2658. doi: 10.1128/jb.174.8.2648-2658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell. 1983 Nov;35(1):275–283. doi: 10.1016/0092-8674(83)90230-1. [DOI] [PubMed] [Google Scholar]