Abstract
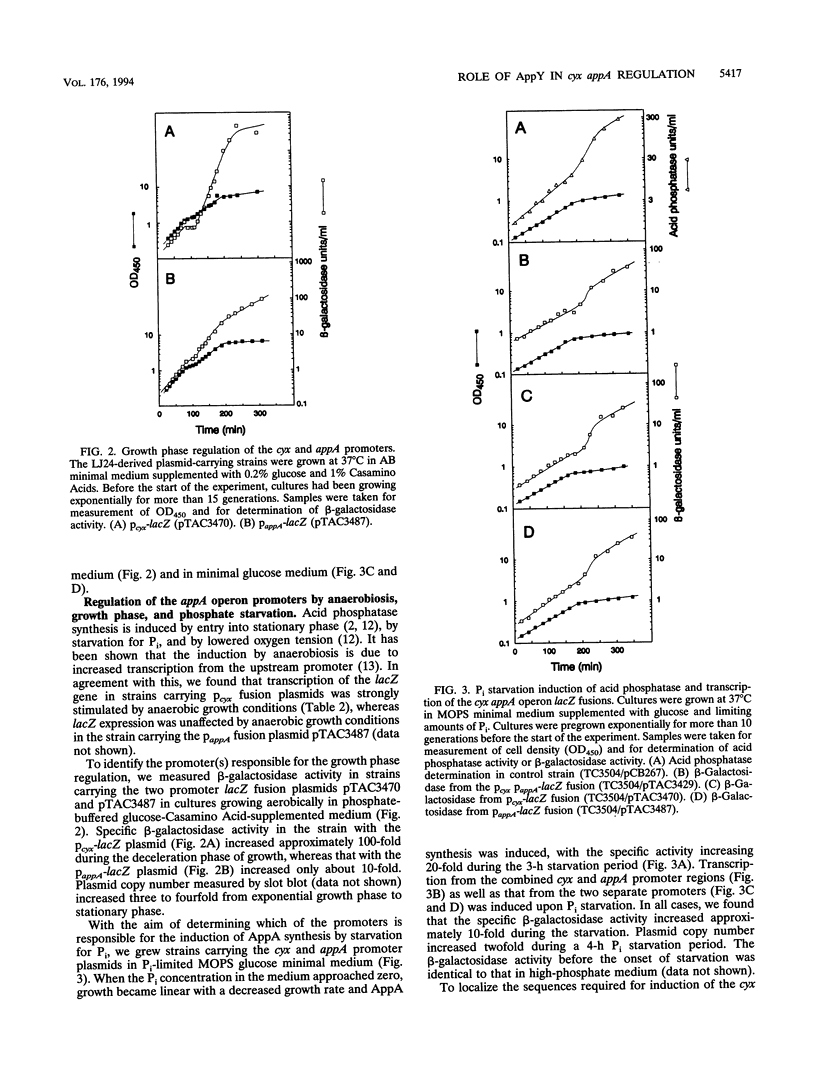
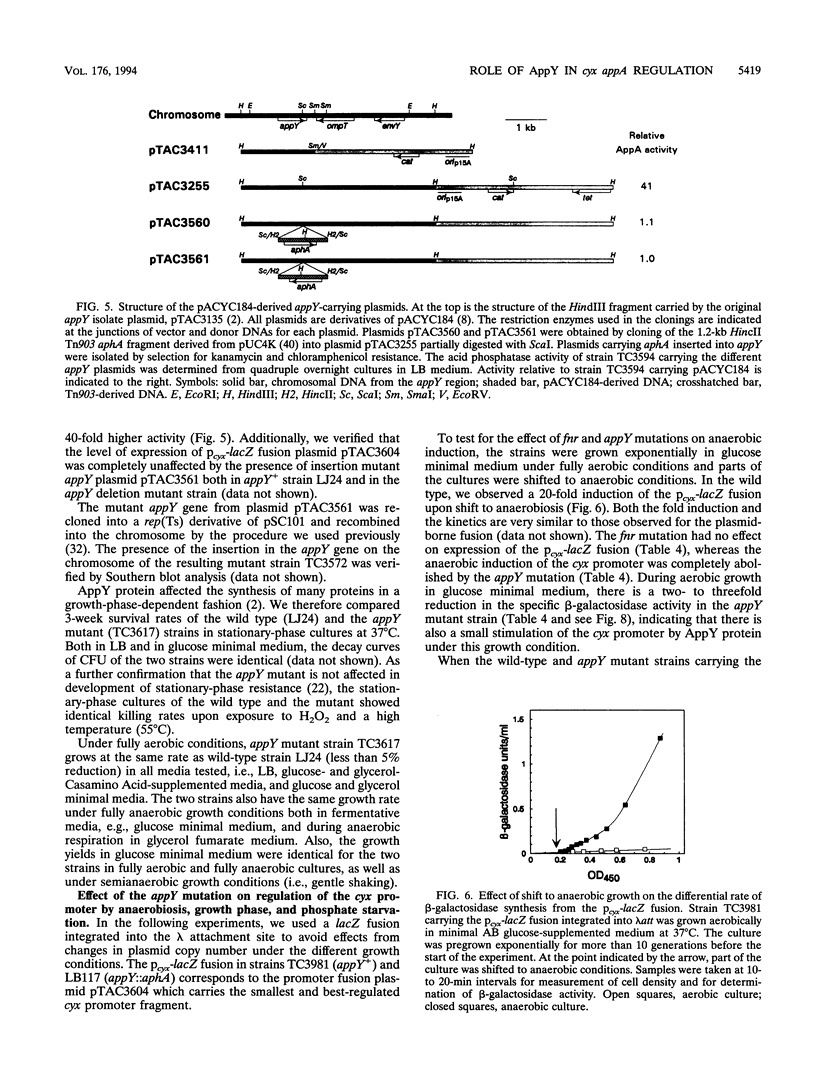
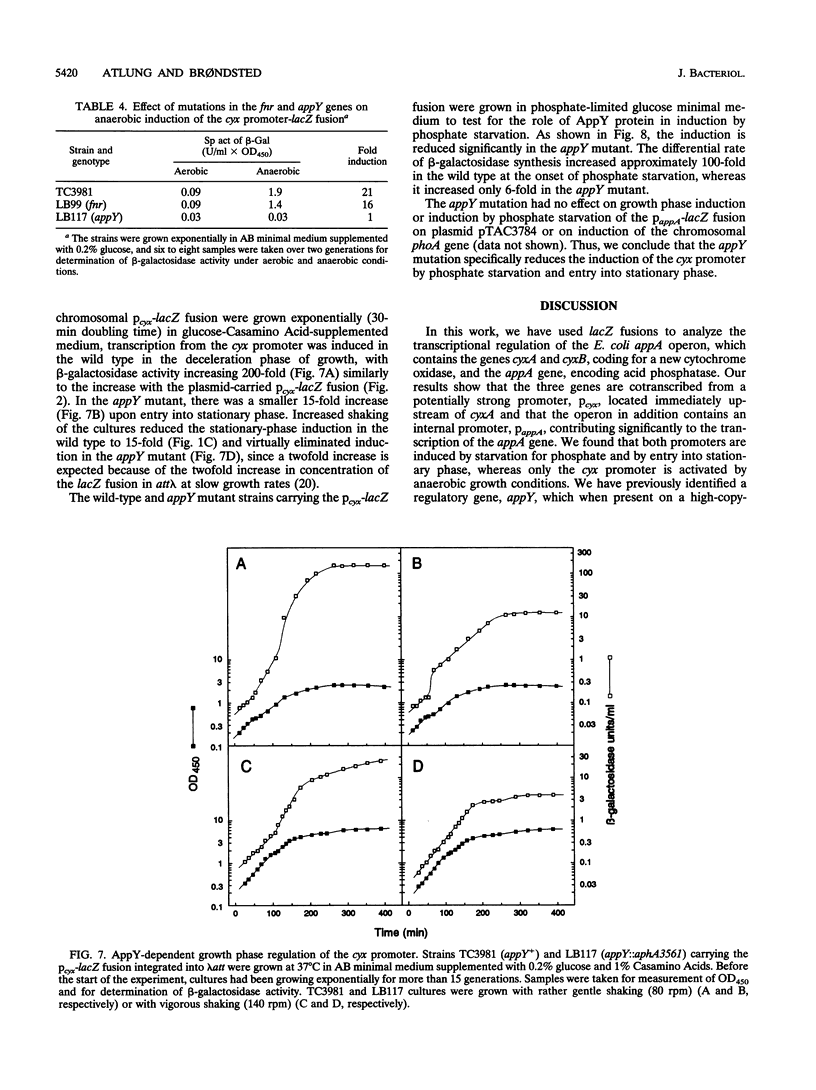
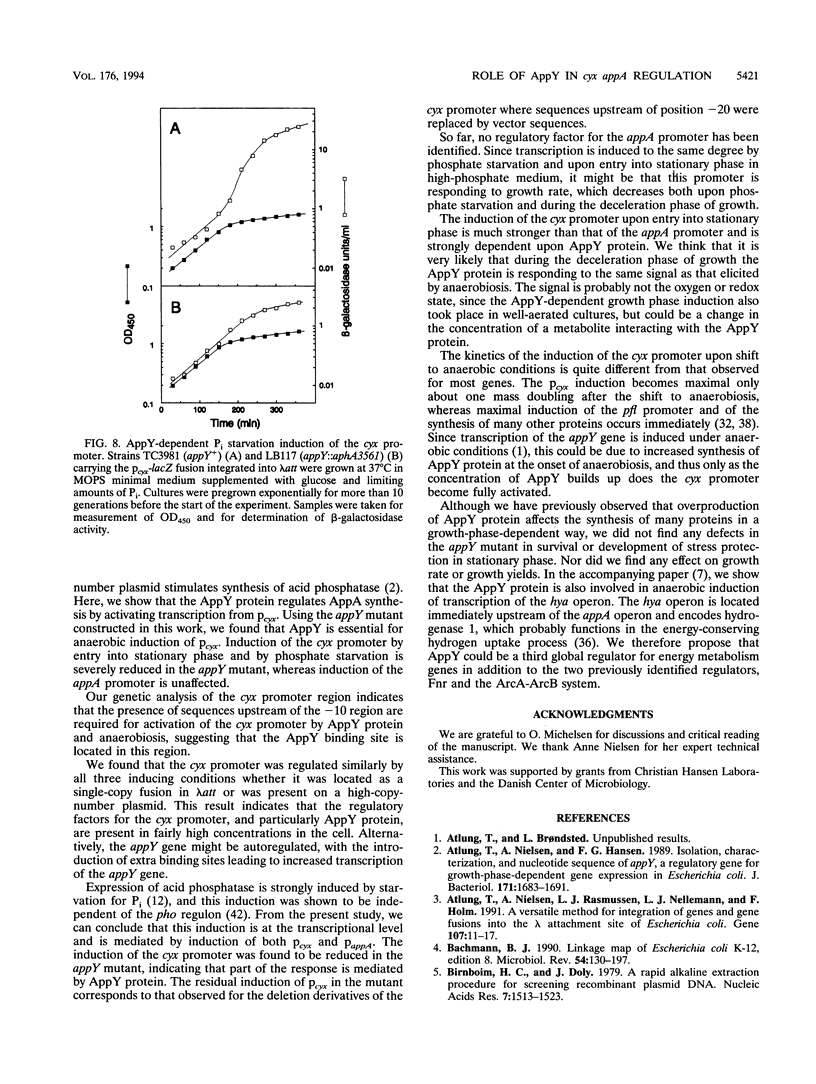
Transcriptional lacZ fusions have been used to analyze the regulation of the appA operon of Escherichia coli. The appA operon contains the genes cyxA and cyxB, coding for the putative third cytochrome oxidase, and appA, encoding acid phosphatase. The analysis showed that the cyxAB and the appA genes are cotranscribed from a potentially strong promoter, Pcyx, located immediately upstream of cyxA and that the operon in addition contains an internal promoter, PappA, contributing significantly to the transcription of the appA gene. The two promoters were both induced by starvation for Pi and by entry into stationary phase. The cyx promoter was in addition found to be activated by anaerobic growth conditions. The product of the previously identified appY gene, which when present on a high-copy-number plasmid stimulates synthesis of acid phosphatase, was shown to activate the cyx promoter. An insertion mutation in the appY gene was constructed in vitro and recombined into the chromosome. The appY mutation eliminated induction of the cyx promoter by anaerobiosis and severely reduced induction of this promoter by phosphate starvation and upon entry into stationary phase but had no effect on induction of the appA promoter. The appY mutation had no effect on survival in stationary phase, nor did it have any effect on growth rate or yield under aerobic or anaerobic conditions. The possibility that AppY is a third global regulator of energy metabolism genes is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlung T., Nielsen A., Hansen F. G. Isolation, characterization, and nucleotide sequence of appY, a regulatory gene for growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1989 Mar;171(3):1683–1691. doi: 10.1128/jb.171.3.1683-1691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlung T., Nielsen A., Rasmussen L. J., Nellemann L. J., Holm F. A versatile method for integration of genes and gene fusions into the lambda attachment site of Escherichia coli. Gene. 1991 Oct 30;107(1):11–17. doi: 10.1016/0378-1119(91)90291-i. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P. L., Manoil C., Beckwith J. Use of TnphoA to detect genes for exported proteins in Escherichia coli: identification of the plasmid-encoded gene for a periplasmic acid phosphatase. J Bacteriol. 1987 Apr;169(4):1663–1669. doi: 10.1128/jb.169.4.1663-1669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brøndsted L., Atlung T. Anaerobic regulation of the hydrogenase 1 (hya) operon of Escherichia coli. J Bacteriol. 1994 Sep;176(17):5423–5428. doi: 10.1128/jb.176.17.5423-5428.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. A., Chepuri V., Gennis R. B., Gunsalus R. P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990 Nov;172(11):6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E., Boquet P. L. Identification of the gene appA for the acid phosphatase (pH optimum 2.5) of Escherichia coli. Mol Gen Genet. 1985;200(1):68–73. doi: 10.1007/BF00383314. [DOI] [PubMed] [Google Scholar]
- Dassa E., Cahu M., Desjoyaux-Cherel B., Boquet P. L. The acid phosphatase with optimum pH of 2.5 of Escherichia coli. Physiological and Biochemical study. J Biol Chem. 1982 Jun 25;257(12):6669–6676. [PubMed] [Google Scholar]
- Dassa J., Fsihi H., Marck C., Dion M., Kieffer-Bontemps M., Boquet P. L. A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA) Mol Gen Genet. 1991 Oct;229(3):341–352. doi: 10.1007/BF00267454. [DOI] [PubMed] [Google Scholar]
- Dassa J., Marck C., Boquet P. L. The complete nucleotide sequence of the Escherichia coli gene appA reveals significant homology between pH 2.5 acid phosphatase and glucose-1-phosphatase. J Bacteriol. 1990 Sep;172(9):5497–5500. doi: 10.1128/jb.172.9.5497-5500.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H. A., Iuchi S., Lin E. C. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991 Apr;226(1-2):209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- Gallegos M. T., Michán C., Ramos J. L. The XylS/AraC family of regulators. Nucleic Acids Res. 1993 Feb 25;21(4):807–810. doi: 10.1093/nar/21.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou C. D., Dueweke T. J., Gennis R. B. Regulation of expression of the cytochrome d terminal oxidase in Escherichia coli is transcriptional. J Bacteriol. 1988 Feb;170(2):961–966. doi: 10.1128/jb.170.2.961-966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner R., Konietzny U., Jany K. D. Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys. 1993 May 15;303(1):107–113. doi: 10.1006/abbi.1993.1261. [DOI] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Atlung T., Braun R. E., Wright A., Hughes P., Kohiyama M. Initiator (DnaA) protein concentration as a function of growth rate in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5194–5199. doi: 10.1128/jb.173.16.5194-5199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Koefoed S., Atlung T. Cloning and nucleotide sequence determination of twelve mutant dnaA genes of Escherichia coli. Mol Gen Genet. 1992 Jul;234(1):14–21. doi: 10.1007/BF00272340. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993 Jan 29;72(2):165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Adaptation of Escherichia coli to respiratory conditions: regulation of gene expression. Cell. 1991 Jul 12;66(1):5–7. doi: 10.1016/0092-8674(91)90130-q. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Earhart C. F. Gene envY of Escherichia coli K-12 affects thermoregulation of major porin expression. J Bacteriol. 1984 Jan;157(1):262–268. doi: 10.1128/jb.157.1.262-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon N. K., Robbins J., Peck H. D., Jr, Chatelus C. Y., Choi E. S., Przybyla A. E. Cloning and sequencing of a putative Escherichia coli [NiFe] hydrogenase-1 operon containing six open reading frames. J Bacteriol. 1990 Apr;172(4):1969–1977. doi: 10.1128/jb.172.4.1969-1977.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L. J., Møller P. L., Atlung T. Carbon metabolism regulates expression of the pfl (pyruvate formate-lyase) gene in Escherichia coli. J Bacteriol. 1991 Oct;173(20):6390–6397. doi: 10.1128/jb.173.20.6390-6397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Böck A. Novel transcriptional control of the pyruvate formate-lyase gene: upstream regulatory sequences and multiple promoters regulate anaerobic expression. J Bacteriol. 1989 May;171(5):2485–2498. doi: 10.1128/jb.171.5.2485-2498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Suppmann B. Anaerobic induction of pyruvate formate-lyase gene expression is mediated by the ArcA and FNR proteins. J Bacteriol. 1992 Jun;174(11):3474–3478. doi: 10.1128/jb.174.11.3474-3478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Jamieson D. J., Higgins C. F., Boxer D. H. Characterization and physiological roles of membrane-bound hydrogenase isoenzymes from Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):398–404. doi: 10.1128/jb.168.1.398-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Beck C. F. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene. 1986;42(1):37–48. doi: 10.1016/0378-1119(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990 Aug;6(4):399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Taylor L. A., Rose R. E. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 1988 Jan 11;16(1):358–358. doi: 10.1093/nar/16.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati E., Danchin A. The structure of the promoter and amino terminal region of the pH 2.5 acid phosphatase structural gene (appA) of E. coli: a negative control of transcription mediated by cyclic AMP. Biochimie. 1987 Mar;69(3):215–221. doi: 10.1016/0300-9084(87)90045-9. [DOI] [PubMed] [Google Scholar]



