Abstract
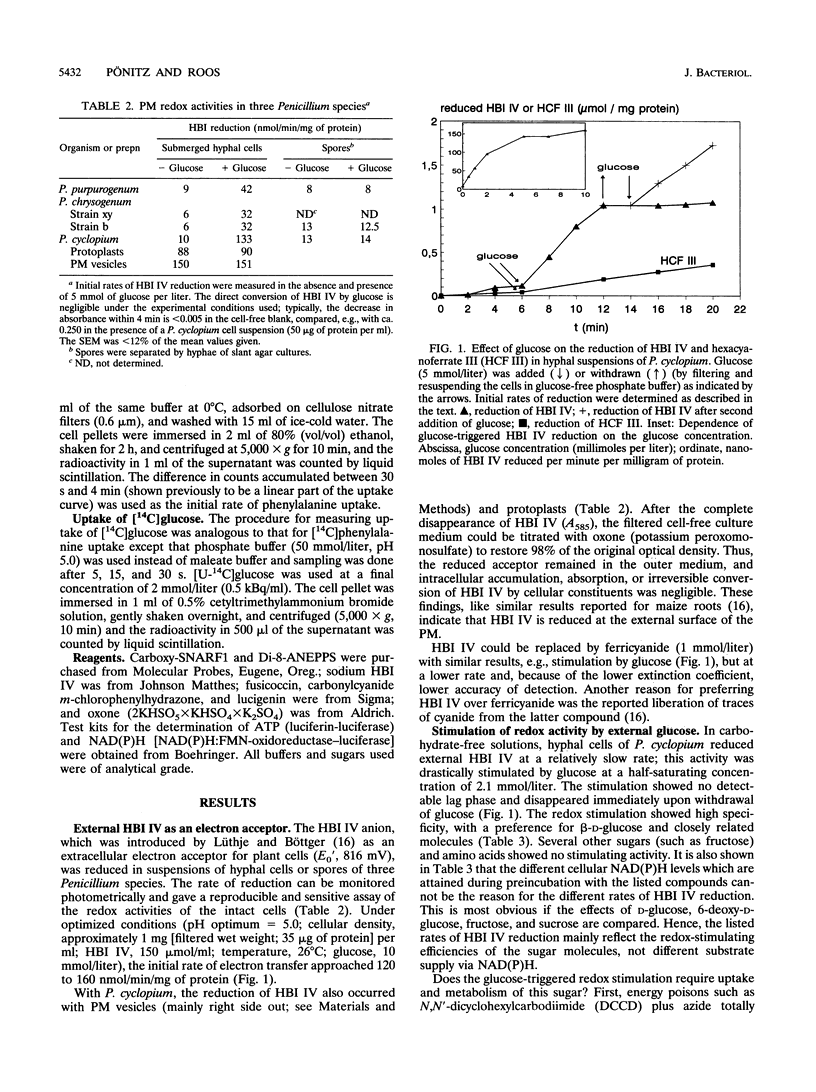
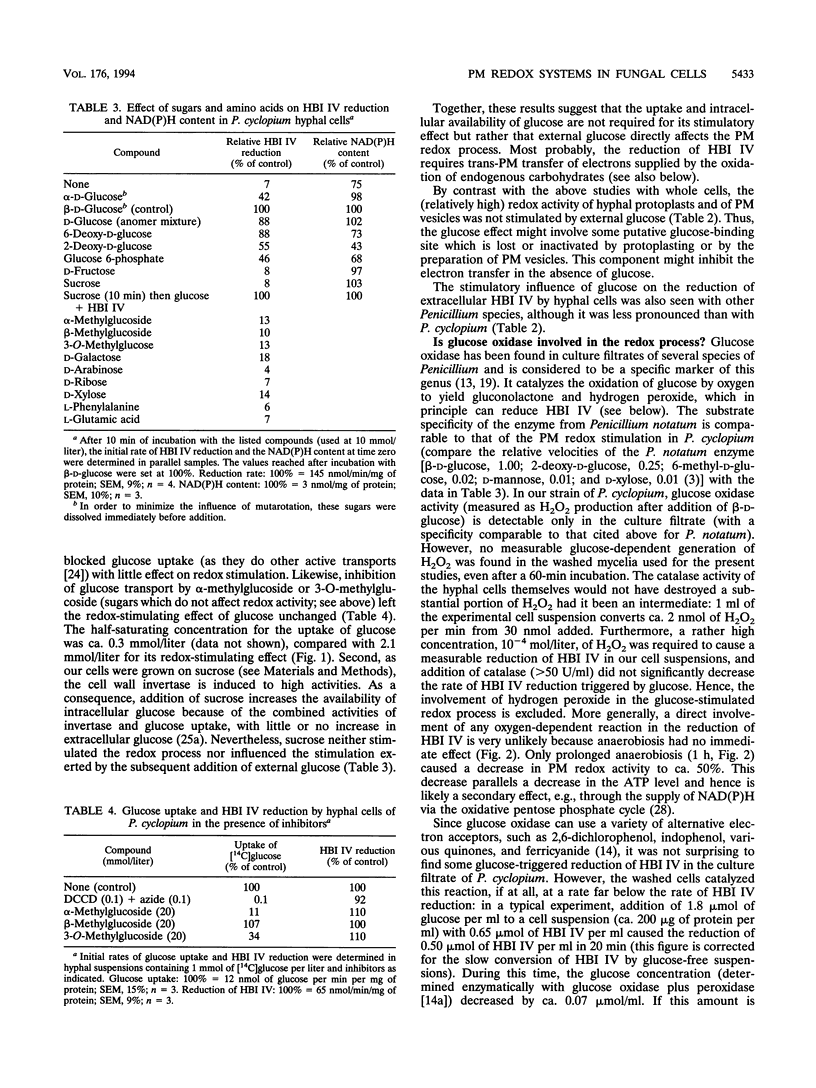
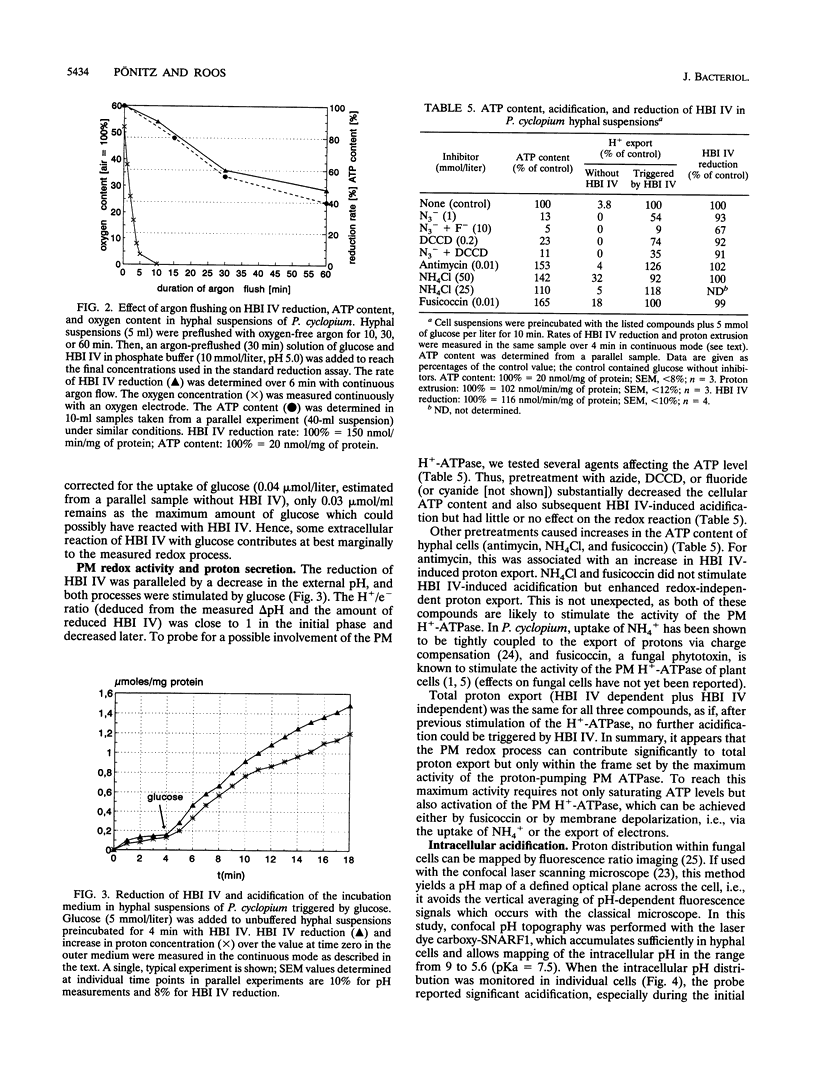
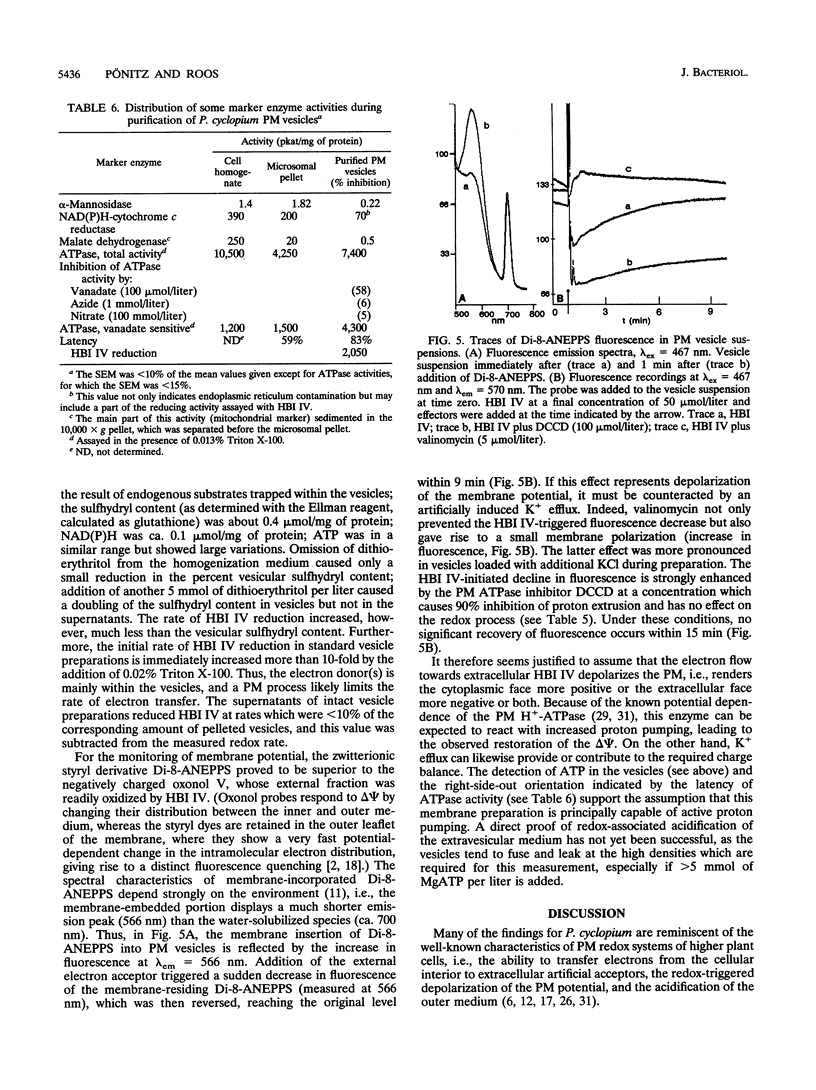
Hyphal cells of three fungal species of the genus Penicillium reduced the nonpermeable, external electron acceptor hexabromoiridate IV (HBI IV). In Penicillium cyclopium, the rate of HBI IV reduction by hyphal cells was drastically increased by the addition of beta-glucose. The stimulation showed high specificity for this sugar and did not require its uptake and cellular metabolism. Cell wall oxidases (e.g., glucose oxidase) did not seem to be involved in the reduction of HBI IV, as no measurable H2O2 was formed from added glucose and removal of oxygen had no effect. We propose that there is a glucose-binding component outside the plasma membrane which controls transmembrane electron fluxes in response to external glucose. Reduction of HBI IV was accompanied by rapid acidification of the cellular interior (measured by confocal pH topography). Subsequently, the outer medium was acidified of the cellular interior (measured by confocal pH topography). Subsequently, the outer medium was acidified with an e-/H+ stoichiometry of > 1. In plasma membrane vesicles containing endogenous electron donors, the membrane-residing fluoroprobe Di-8-ANEPPS reported a transient depolarization of the membrane potential triggered by the external electron acceptor. Inhibitors of ATP-dependent proton pumping enhanced the extent of this depolarization, inhibited the subsequent normalization of membrane potential, and, in whole cells, reduced the amount of redox-triggered proton extrusion. From these and other findings, it is concluded that the observed trans-plasma membrane redox process activates the H(+)-ATPase via membrane depolarization and cytosolic acidification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Bersch B. Oxonol VI as an optical indicator for membrane potentials in lipid vesicles. Biochim Biophys Acta. 1987 Oct 16;903(3):480–494. doi: 10.1016/0005-2736(87)90055-1. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Dow J. M., Carreon R. R., Villa V. D. Role of membranes of mycelial Mucor rouxii in synthesis and secretion of cell wall matrix polymers. J Bacteriol. 1981 Jan;145(1):272–279. doi: 10.1128/jb.145.1.272-279.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhler E., Burnham V. G., Loew L. M. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985 Oct 8;24(21):5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Nakamura S. Comparison of fungal glucose oxidases. Chemical, physicochemical and immunological studies. Biochim Biophys Acta. 1976 Jun 7;438(1):37–48. doi: 10.1016/0005-2744(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Montana V., Farkas D. L., Loew L. M. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry. 1989 May 30;28(11):4536–4539. doi: 10.1021/bi00437a003. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G. An H-ATPase Assay: Proton Pumping and ATPase Activity Determined Simultaneously in the Same Sample. Plant Physiol. 1990 Nov;94(3):882–886. doi: 10.1104/pp.94.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncal T., Ugalde U. O., Irastorza A. Calcium-induced conidiation in Penicillium cyclopium: calcium triggers cytosolic alkalinization at the hyphal tip. J Bacteriol. 1993 Feb;175(3):879–886. doi: 10.1128/jb.175.3.879-886.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W. Kinetic properties, nutrient-dependent regulation and energy coupling of amino-acid transport systems in Penicillium cyclopium. Biochim Biophys Acta. 1989 Jan 16;978(1):119–133. doi: 10.1016/0005-2736(89)90507-5. [DOI] [PubMed] [Google Scholar]
- Roos W., Slavík J. Intracellular pH topography of Penicillium cyclopium protoplasts. Maintenance of delta pH by both passive and active mechanisms. Biochim Biophys Acta. 1987 May 12;899(1):67–75. doi: 10.1016/0005-2736(87)90240-9. [DOI] [PubMed] [Google Scholar]
- Singh M., Scrutton N. S., Scrutton M. C. NADPH generation in Aspergillus nidulans: is the mannitol cycle involved? J Gen Microbiol. 1988 Mar;134(3):643–654. doi: 10.1099/00221287-134-3-643. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. The plasma membrane ATPase of Neurospora: a proton-pumping electroenzyme. J Bioenerg Biomembr. 1987 Feb;19(1):1–20. doi: 10.1007/BF00769728. [DOI] [PubMed] [Google Scholar]
- Sollod C. C., Jenns A. E., Daub M. E. Cell surface redox potential as a mechanism of defense against photosensitizers in fungi. Appl Environ Microbiol. 1992 Feb;58(2):444–449. doi: 10.1128/aem.58.2.444-449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde U. O., Hernandez A., Galindo I., Pitt D., Barnes J. C., Wakley G. Preparation of right-side-out plasma membrane vesicles from Penicillium cyclopium: a critical assessment of markers. J Gen Microbiol. 1992 Oct;138(10):2205–2212. doi: 10.1099/00221287-138-10-2205. [DOI] [PubMed] [Google Scholar]
- Yamashoji S., Kajimoto G. Decrease of NADH in yeast cells by external ferricyanide reduction. Biochim Biophys Acta. 1986 Nov 5;852(1):25–29. doi: 10.1016/0005-2728(86)90052-6. [DOI] [PubMed] [Google Scholar]



