Abstract
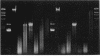
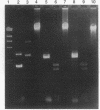
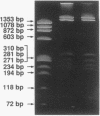
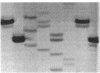
In the process of developing a gene transfer system for the marine, unicellular, nitrogen-fixing cyanobacterium Cyanothece sp. strain BH68K, two major restriction barriers have been identified. A cell wall-associated nuclease exhibited non-site-specific degradation of covalently closed circular and linear double-stranded DNA molecules, including Cyanothece sp. strain BH68K chromosomal DNA. The nuclease is easily released from intact cells by using water or buffer containing Triton X-100. Nuclease activity was undetectable in cell extracts prepared from water-washed cells. Comparison of the restriction endonuclease susceptibility of Cyanothece sp. strain BH68K DNA to that of Anabaena sp. strain PCC 7120 revealed that these organisms have a nearly identical pattern of restriction and therefore may contain similar systems for DNA methylation. Restriction by DpnI, MboI, and Sau3AI indicated the presence of adenine methylation. Cyanothece sp. strain BH68K cell extracts contain a type II restriction endonuclease, Csp68KI. The activity of Csp68KI was easily detected in cell extracts without extensive purification. Csp68KI is an isoschizomer of AvaII and recognizes the nucleotide sequence 5'-GG(A/T)CC-3'. Cleavage occurs between the guanosine nucleotides producing 3-bp 5' overhang ends.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. G. Isolation and restriction analysis of DNA from heterocysts and vegetative cells of cyanobacteria. J Gen Microbiol. 1988 Nov;134(11):2943–2949. doi: 10.1099/00221287-134-11-2943. [DOI] [PubMed] [Google Scholar]
- Bancroft I., Smith R. J. An analysis of restriction endonuclease sites in cyanophages infecting the heterocystous cyanobacteria Anabaena and Nostoc. J Gen Virol. 1988 Mar;69(Pt 3):739–743. doi: 10.1099/0022-1317-69-3-739. [DOI] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P., Oren E. V. Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Nov;171(11):5940–5948. doi: 10.1128/jb.171.11.5940-5948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle T. A., Krüger D. H. Biology of DNA restriction. Microbiol Rev. 1993 Jun;57(2):434–450. doi: 10.1128/mr.57.2.434-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brooks J. E., Blumenthal R. M., Gingeras T. R. The isolation and characterization of the Escherichia coli DNA adenine methylase (dam) gene. Nucleic Acids Res. 1983 Feb 11;11(3):837–851. doi: 10.1093/nar/11.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. L., Smith M. A general method for defining restriction enzyme cleavage and recognition sites. Methods Enzymol. 1980;65(1):391–404. doi: 10.1016/s0076-6879(80)65050-2. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Focareta T., Manning P. A. Distinguishing between the extracellular DNases of Vibrio cholerae and development of a transformation system. Mol Microbiol. 1991 Oct;5(10):2547–2555. doi: 10.1111/j.1365-2958.1991.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Hughes S. G., Murray K. The nucleotide sequences recognized by endonucleases AvaI and AvaII from Anabaena variabilis. Biochem J. 1980 Jan 1;185(1):65–75. doi: 10.1042/bj1850065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger K., Potts M. Distinct fractions of genomic DNA from cyanobacterium Nostoc commune that differ in the degree of methylation. Gene. 1988 Dec 25;74(1):197–201. doi: 10.1016/0378-1119(88)90286-7. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C. J., van Arkel G. A. Host-vector systems for gene cloning in cyanobacteria. Methods Enzymol. 1987;153:199–215. doi: 10.1016/0076-6879(87)53054-3. [DOI] [PubMed] [Google Scholar]
- Lambert G. R., Carr N. G. Resistance of DNA from filamentous and unicellular cyanobacteria to restriction endonuclease cleavage. Biochim Biophys Acta. 1984 Feb 24;781(1-2):45–55. doi: 10.1016/0167-4781(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Muro-Pastor A. M., Flores E., Herrero A., Wolk C. P. Identification, genetic analysis and characterization of a sugar-non-specific nuclease from the cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 1992 Oct;6(20):3021–3030. doi: 10.1111/j.1365-2958.1992.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Nelson M., Raschke E., McClelland M. Effect of site-specific methylation on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res. 1993 Jul 1;21(13):3139–3154. doi: 10.1093/nar/21.13.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhy R. N., Hottat F. G., Coene M. M., Hoet P. P. Restriction analysis and quantitative estimation of methylated bases of filamentous and unicellular cyanobacterial DNAs. J Bacteriol. 1988 Apr;170(4):1934–1939. doi: 10.1128/jb.170.4.1934-1939.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. J., Haskell J. B., Sherman D. M., Sherman L. A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J Bacteriol. 1993 Mar;175(5):1284–1292. doi: 10.1128/jb.175.5.1284-1292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N. A new revision of the sequence of plasmid pBR322. Gene. 1988 Oct 30;70(2):399–403. doi: 10.1016/0378-1119(88)90212-0. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zehr J. P., Ohki K., Fujita Y., Landry D. Unique modification of adenine in genomic DNA of the marine cyanobacterium Trichodesmium sp. strain NIBB 1067. J Bacteriol. 1991 Nov;173(21):7059–7062. doi: 10.1128/jb.173.21.7059-7062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]