Abstract
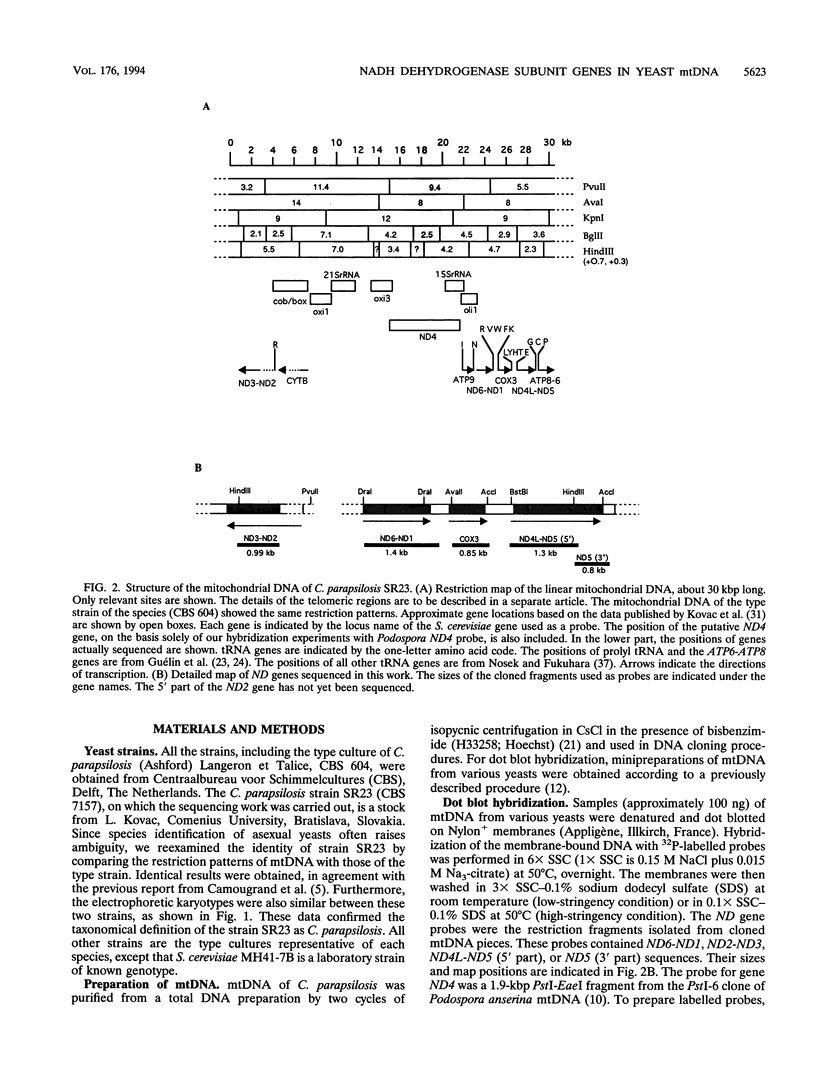
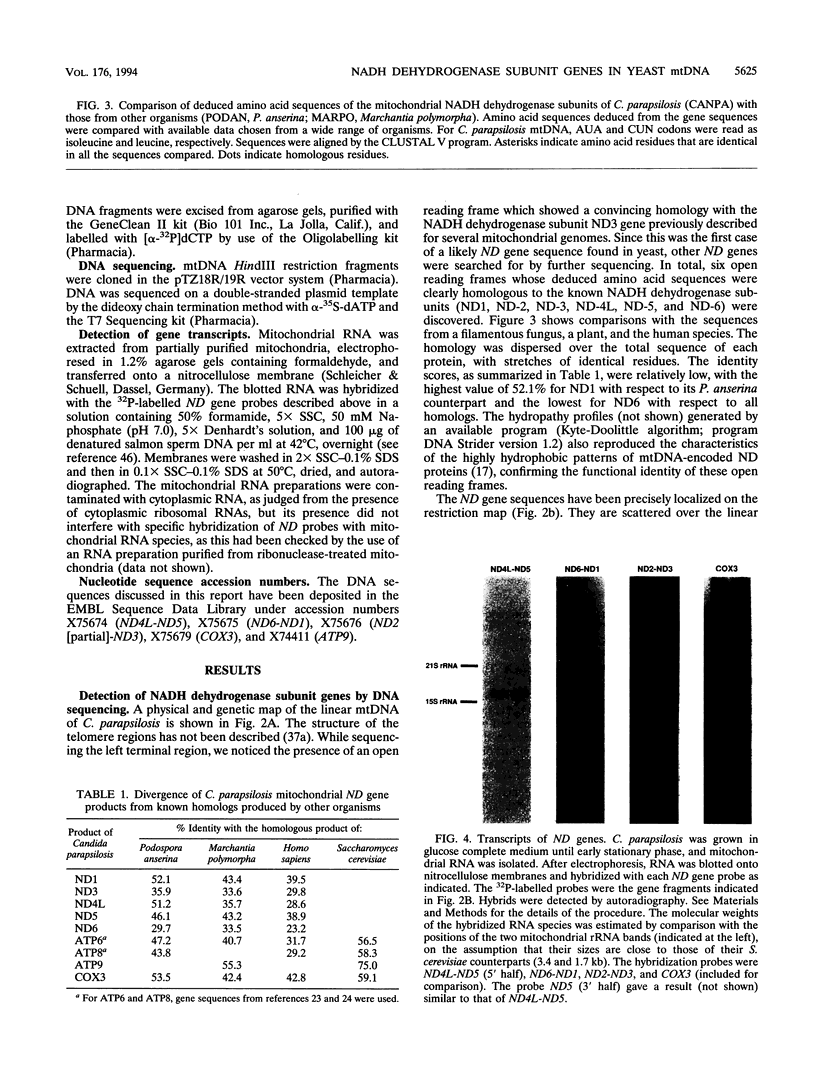
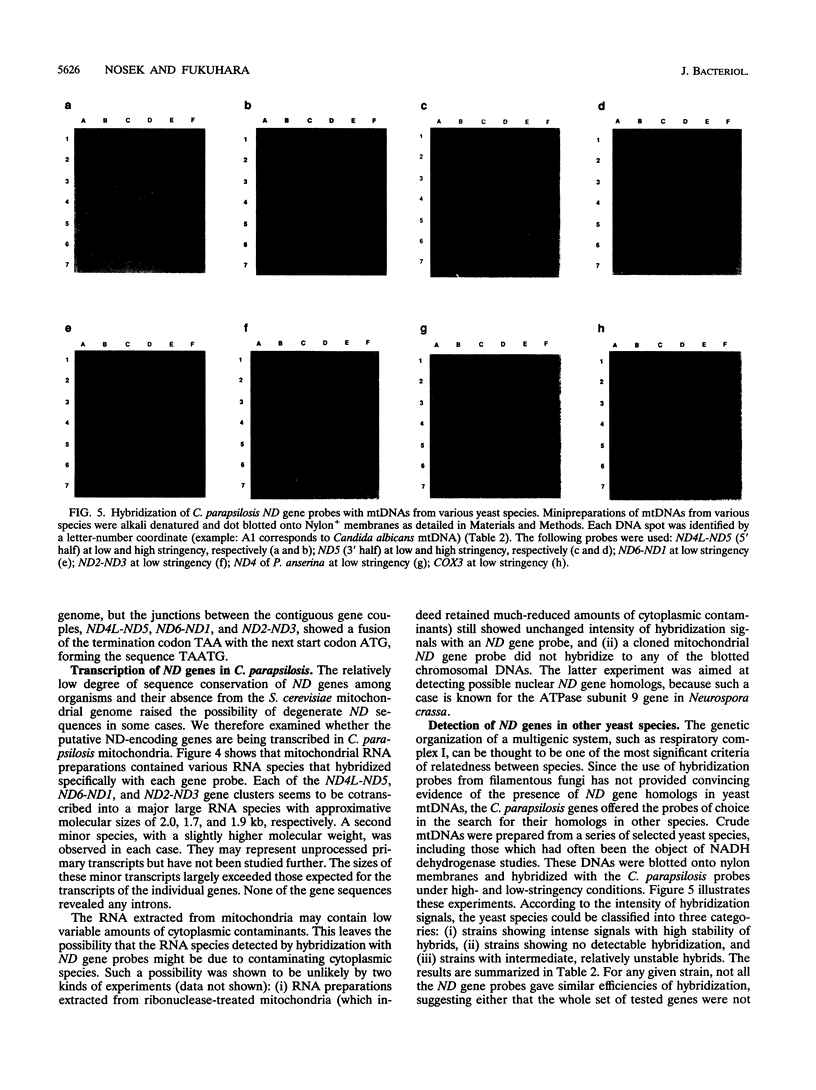
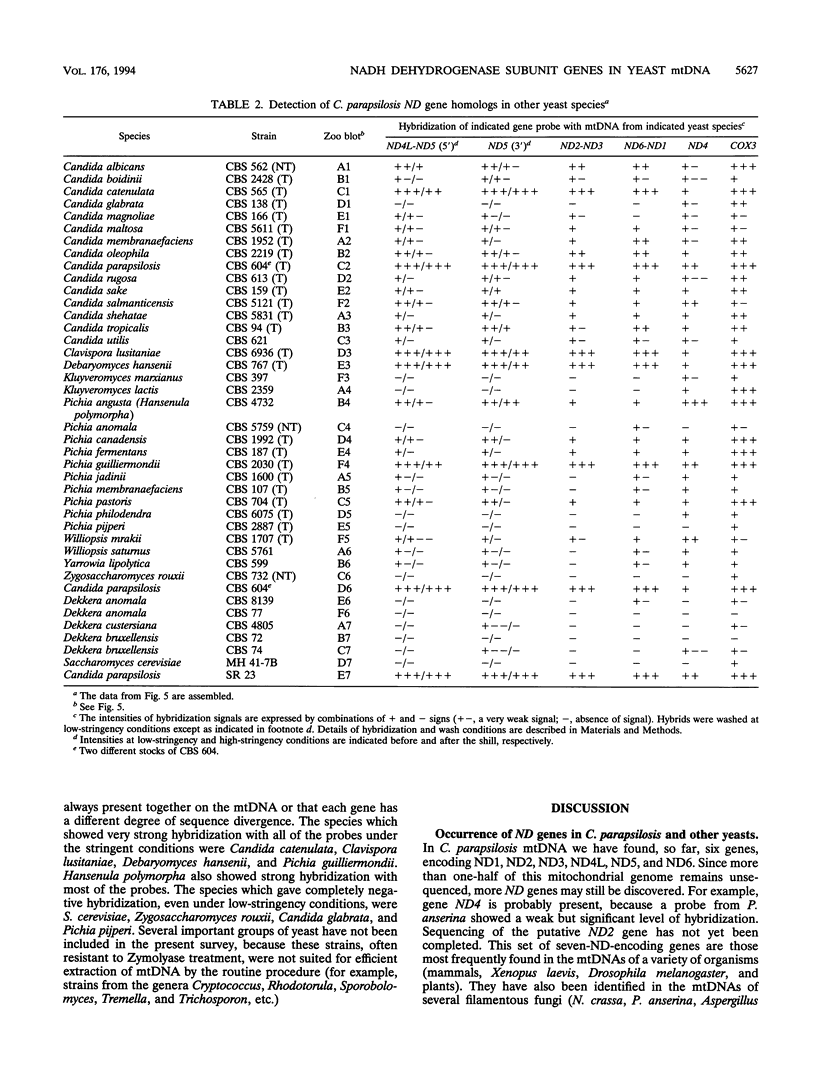
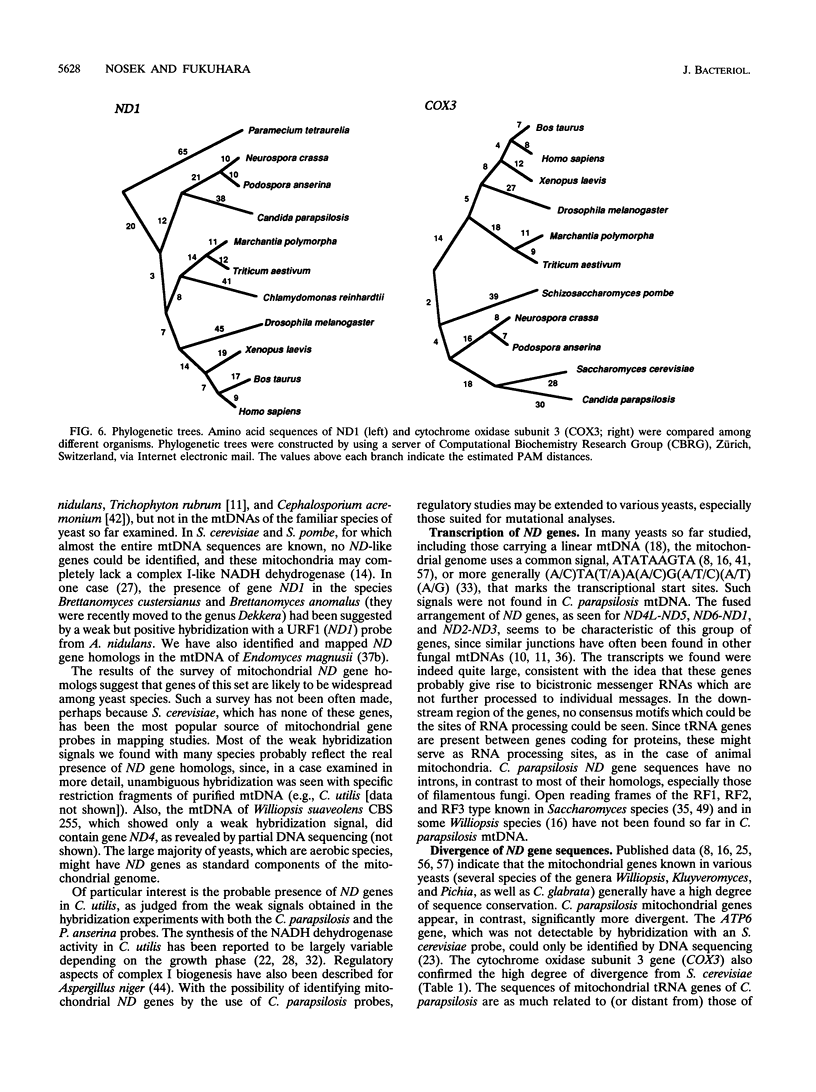
The genes encoding the NADH dehydrogenase subunits of respiratory complex I have not been identified so far in the mitochondrial DNA (mtDNA) of yeasts. In the linear mtDNA of Candida parapsilosis, we found six new open reading frames whose sequences were unambiguously homologous to those of the genes known to code for NADH dehydrogenase subunit proteins of different organisms, i.e., ND1, ND2, ND3, ND4L, ND5, and ND6. The gene for ND4 also appears to be present, as judged from hybridization experiments with a Podospora gene probe. Specific transcripts from these open reading frames (ND genes) could be detected in the mitochondria. Hybridization experiments using C. parapsilosis genes as probes suggested that ND genes are present in the mtDNAs of a wide range of yeast species including Candida catenulata, Pichia guilliermondii, Clavispora lusitaniae, Debaryomyces hansenii, Hansenula polymorpha, and others.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. The Aspergillus nidulans mitochondrial genome. Curr Genet. 1985;9(2):113–117. doi: 10.1007/BF00436957. [DOI] [PubMed] [Google Scholar]
- Camougrand N., Mila B., Velours G., Lazowska J., Guérin M. Discrimination between different groups of Candida parapsilosis by mitochondrial DNA restriction analysis. Curr Genet. 1988 May;13(5):445–449. doi: 10.1007/BF00365667. [DOI] [PubMed] [Google Scholar]
- Camougrand N., Velours G., Guerin M. The energetic growth yields of the yeast Candida parapsilosis. Biol Cell. 1987;61(3):171–175. doi: 10.1111/j.1768-322x.1987.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Goddard J. M., Martin S. C., Fauron C. M., Wolstenholme D. R. Drosophila mitochondrial DNA: a novel gene order. Nucleic Acids Res. 1982 Nov 11;10(21):6619–6637. doi: 10.1093/nar/10.21.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., McNally K. L., Domenico J. M., Matsuura E. T. The complete DNA sequence of the mitochondrial genome of Podospora anserina. Curr Genet. 1990 May;17(5):375–402. doi: 10.1007/BF00334517. [DOI] [PubMed] [Google Scholar]
- Defontaine A., Lecocq F. M., Hallet J. N. A rapid miniprep method for the preparation of yeast mitochondrial DNA. Nucleic Acids Res. 1991 Jan 11;19(1):185–185. doi: 10.1093/nar/19.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drissi R., Sor F., Nosek J., Fukuhara H. Genes of the linear mitochondrial DNA of Williopsis mrakii: coding sequences for a maturase-like protein, a ribosomal protein VAR1 homologue, cytochrome oxidase subunit 2 and methionyl tRNA. Yeast. 1994 Mar;10(3):391–398. doi: 10.1002/yea.320100312. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim Biophys Acta. 1992 Dec 7;1140(2):105–134. doi: 10.1016/0005-2728(92)90001-i. [DOI] [PubMed] [Google Scholar]
- Fukuhara H., Sor F., Drissi R., Dinouël N., Miyakawa I., Rousset S., Viola A. M. Linear mitochondrial DNAs of yeasts: frequency of occurrence and general features. Mol Cell Biol. 1993 Apr;13(4):2309–2314. doi: 10.1128/mcb.13.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M., Azoulay E. Métabolisme énergétique chez Candida tropicalis. Oxydation phosphorylante chez Candida tropicalis cultivé sur alcanes. Biochimie. 1974;56(8):1129–1143. doi: 10.1016/s0300-9084(74)80101-x. [DOI] [PubMed] [Google Scholar]
- Garesse R. Drosophila melanogaster mitochondrial DNA: gene organization and evolutionary considerations. Genetics. 1988 Apr;118(4):649–663. doi: 10.1093/genetics/118.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S., Cobley J. G., Singer T. P. Reduced nicotinamide adenine dinucleotide dehydrogenase, piericidin sensitivity, and site 1 phosphorylation in different growth phases of Candida utilis. J Biol Chem. 1974 Jun 25;249(12):3819–3826. [PubMed] [Google Scholar]
- Guelin E., Velours J., Guerin M. Cloning and sequencing of a fragment of the linear mitochondrial DNA of the yeast Candida parapsilosis supporting genes encoding subunit 8 of Fo ATP synthase and a putative t-RNA(Pro). Nucleic Acids Res. 1990 Jul 25;18(14):4267–4267. doi: 10.1093/nar/18.14.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guélin E., Guérin M., Velours J. Isolation of the ATP synthase subunit 6 and sequence of the mitochondrial ATP6 gene of the yeast Candida parapsilosis. Eur J Biochem. 1991 Apr 10;197(1):105–111. doi: 10.1111/j.1432-1033.1991.tb15887.x. [DOI] [PubMed] [Google Scholar]
- Hardy C. M., Clark-Walker G. D. Nucleotide sequence of the cytochrome oxidase subunit 2 and val-tRNA genes and surrounding sequences from Kluyveromyces lactis K8 mitochondrial DNA. Yeast. 1990 Sep-Oct;6(5):403–410. doi: 10.1002/yea.320060505. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hoeben P., Clark-Walker G. D. An approach to yeast classification by mapping mitochondrial DNA from Dekkera/Brettanomyces and Eeniella genera. Curr Genet. 1986;10(5):371–379. doi: 10.1007/BF00418409. [DOI] [PubMed] [Google Scholar]
- Katz R., Kilpatrick L., Chance B. Acquisition and loss of rotenone sensitivity in Torulopsis utilis. Eur J Biochem. 1971 Aug 16;21(3):301–307. doi: 10.1111/j.1432-1033.1971.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Koslowsky D. J., Bhat G. J., Perrollaz A. L., Feagin J. E., Stuart K. The MURF3 gene of T. brucei contains multiple domains of extensive editing and is homologous to a subunit of NADH dehydrogenase. Cell. 1990 Sep 7;62(5):901–911. doi: 10.1016/0092-8674(90)90265-g. [DOI] [PubMed] [Google Scholar]
- Kovác L., Lazowska J., Slonimski P. P. A yeast with linear molecules of mitochondrial DNA. Mol Gen Genet. 1984;197(3):420–424. doi: 10.1007/BF00329938. [DOI] [PubMed] [Google Scholar]
- Marczynski G. T., Schultz P. W., Jaehning J. A. Use of yeast nuclear DNA sequences to define the mitochondrial RNA polymerase promoter in vitro. Mol Cell Biol. 1989 Aug;9(8):3193–3202. doi: 10.1128/mcb.9.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis G., Vahrenholz C., Pratje E. Mitochondrial DNA of Chlamydomonas reinhardtii: the gene for apocytochrome b and the complete functional map of the 15.8 kb DNA. Mol Gen Genet. 1990 Sep;223(2):211–216. doi: 10.1007/BF00265056. [DOI] [PubMed] [Google Scholar]
- Nelson M. A., Macino G. Structure and expression of the overlapping ND4L and ND5 genes of Neurospora crassa mitochondria. Mol Gen Genet. 1987 Feb;206(2):307–317. doi: 10.1007/BF00333589. [DOI] [PubMed] [Google Scholar]
- Nosek J., Fukuhara H. Mitochondrial transfer RNA genes of the yeast Candida parapsilosis. Gene. 1994 May 16;142(2):307–308. doi: 10.1016/0378-1119(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Oda K., Yamato K., Ohta E., Nakamura Y., Takemura M., Nozato N., Akashi K., Kanegae T., Ogura Y., Kohchi T. Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. A primitive form of plant mitochondrial genome. J Mol Biol. 1992 Jan 5;223(1):1–7. doi: 10.1016/0022-2836(92)90708-r. [DOI] [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Osinga K. A., De Haan M., Christianson T., Tabak H. F. A nonanucleotide sequence involved in promotion of ribosomal RNA synthesis and RNA priming of DNA replication in yeast mitochondria. Nucleic Acids Res. 1982 Dec 20;10(24):7993–8006. doi: 10.1093/nar/10.24.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., García J. L. The subunit I of the respiratory-chain NADH dehydrogenase from Cephalosporium acremonium: the evolution of a mitochondrial gene. Curr Genet. 1986;10(11):797–801. doi: 10.1007/BF00418525. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Mahalingam R., Sable C. L., Venuti S. E., Cummings D. J. Nucleotide sequence of the mitochondrial genome of Paramecium. Nucleic Acids Res. 1990 Jan 11;18(1):173–180. doi: 10.1093/nar/18.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prömper C., Schneider R., Weiss H. The role of the proton-pumping and alternative respiratory chain NADH:ubiquinone oxidoreductases in overflow catabolism of Aspergillus niger. Eur J Biochem. 1993 Aug 15;216(1):223–230. doi: 10.1111/j.1432-1033.1993.tb18136.x. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Sankoff D., Leduc G., Antoine N., Paquin B., Lang B. F., Cedergren R. Gene order comparisons for phylogenetic inference: evolution of the mitochondrial genome. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6575–6579. doi: 10.1073/pnas.89.14.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Racker E. Stable phosphorylating submitochondrial particles from baker's yeast. Biochem Biophys Res Commun. 1966 Mar 8;22(5):579–584. doi: 10.1016/0006-291x(66)90314-7. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonimski P. P., Brouillet S. A data-base of chromosome III of Saccharomyces cerevisiae. Yeast. 1993 Sep;9(9):941–1029. doi: 10.1002/yea.320090902. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Analysis of chromosomal DNA patterns of the genus Kluyveromyces. Yeast. 1989 Jan-Feb;5(1):1–10. doi: 10.1002/yea.320050103. [DOI] [PubMed] [Google Scholar]
- Souza A. E., Shu H. H., Read L. K., Myler P. J., Stuart K. D. Extensive editing of CR2 maxicircle transcripts of Trypanosoma brucei predicts a protein with homology to a subunit of NADH dehydrogenase. Mol Cell Biol. 1993 Nov;13(11):6832–6840. doi: 10.1128/mcb.13.11.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M. The chloroplast chromosomes in land plants. Annu Rev Cell Biol. 1989;5:51–70. doi: 10.1146/annurev.cb.05.110189.000411. [DOI] [PubMed] [Google Scholar]
- Séraphin B., Simon M., Faye G. The mitochondrial reading frame RF3 is a functional gene in Saccharomyces uvarum. J Biol Chem. 1987 Jul 25;262(21):10146–10153. [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wilson C., Ragnini A., Fukuhara H. Analysis of the regions coding for transfer RNAs in Kluyveromyces lactis mitochondrial DNA. Nucleic Acids Res. 1989 Jun 26;17(12):4485–4491. doi: 10.1093/nar/17.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bièvre C., Dujon B. Mitochondrial DNA sequence analysis of the cytochrome oxidase subunit I and II genes, the ATPase9 gene, the NADH dehydrogenase ND4L and ND5 gene complex, and the glutaminyl, methionyl and arginyl tRNA genes from Trichophyton rubrum. Curr Genet. 1992 Sep;22(3):229–234. doi: 10.1007/BF00351730. [DOI] [PubMed] [Google Scholar]
- de Vries H., Alzner-DeWeerd B., Breitenberger C. A., Chang D. D., de Jonge J. C., RajBhandary U. L. The E35 stopper mutant of Neurospora crassa: precise localization of deletion endpoints in mitochondrial DNA and evidence that the deleted DNA codes for a subunit of NADH dehydrogenase. EMBO J. 1986 Apr;5(4):779–785. doi: 10.1002/j.1460-2075.1986.tb04281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries S., Grivell L. A. Purification and characterization of a rotenone-insensitive NADH:Q6 oxidoreductase from mitochondria of Saccharomyces cerevisiae. Eur J Biochem. 1988 Sep 15;176(2):377–384. doi: 10.1111/j.1432-1033.1988.tb14292.x. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Bernardi G. The primary structure of the mitochondrial genome of Saccharomyces cerevisiae--a review. Gene. 1986;47(2-3):155–177. doi: 10.1016/0378-1119(86)90060-0. [DOI] [PubMed] [Google Scholar]