Abstract
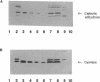
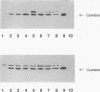
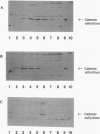
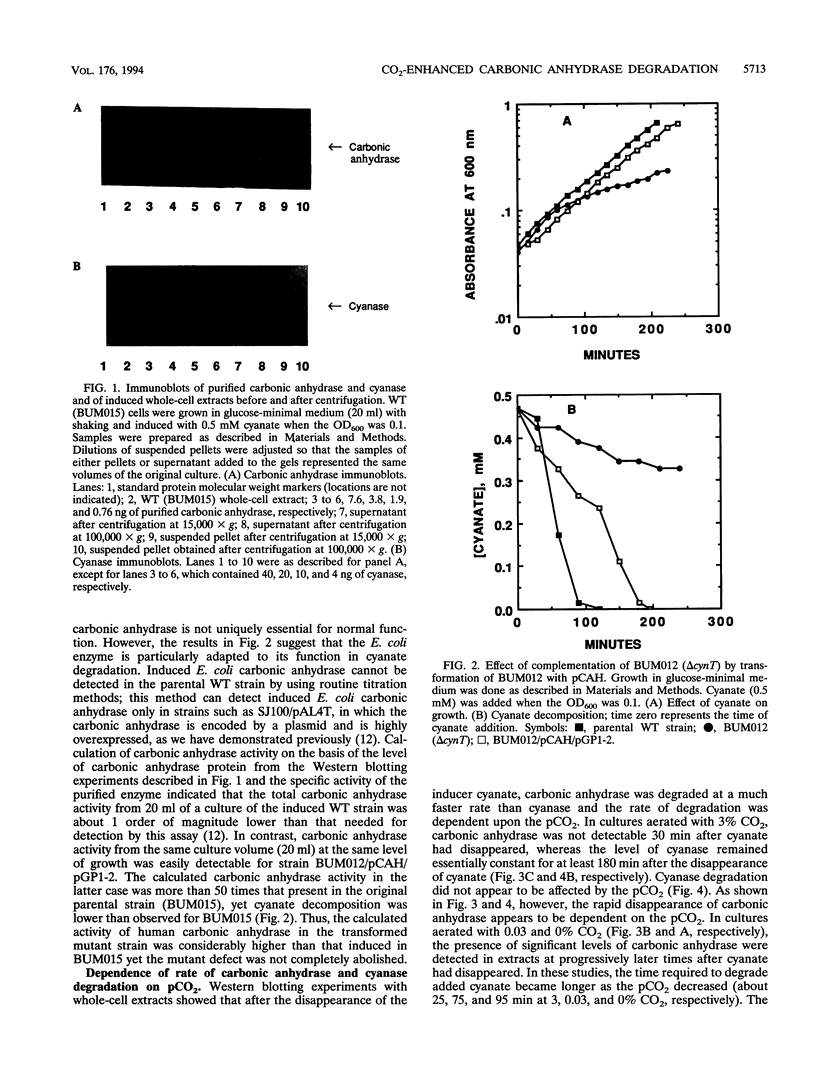
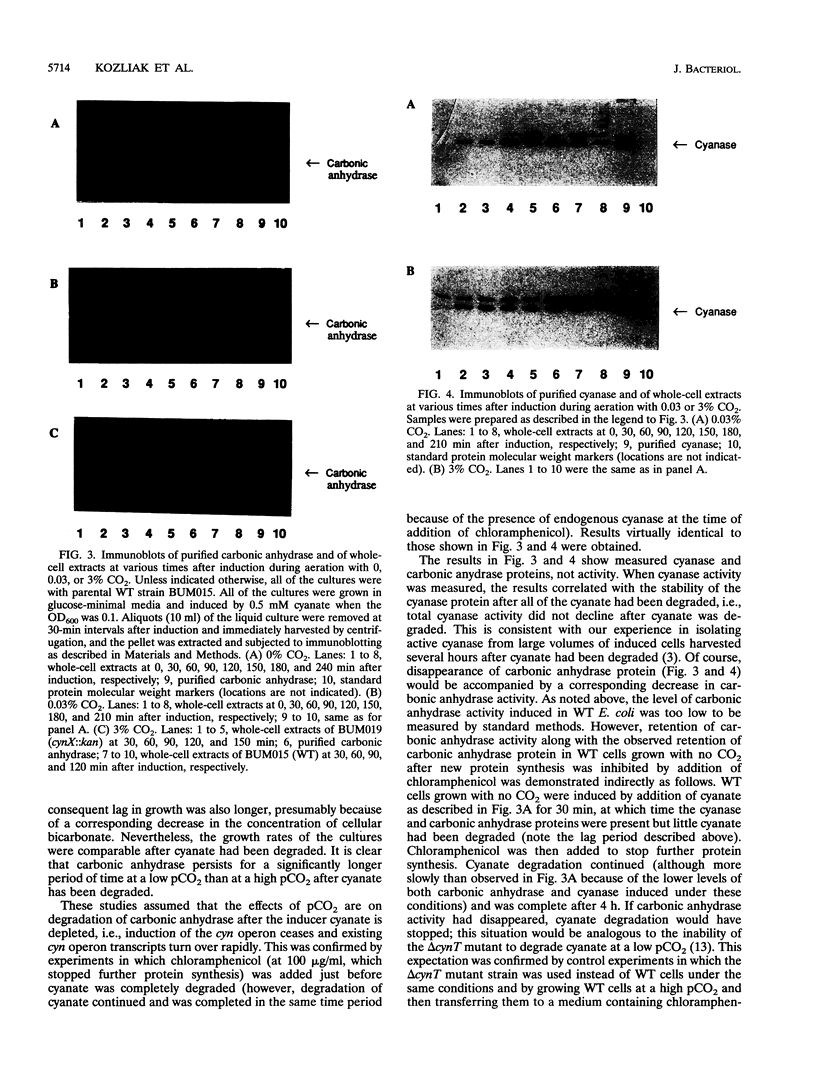
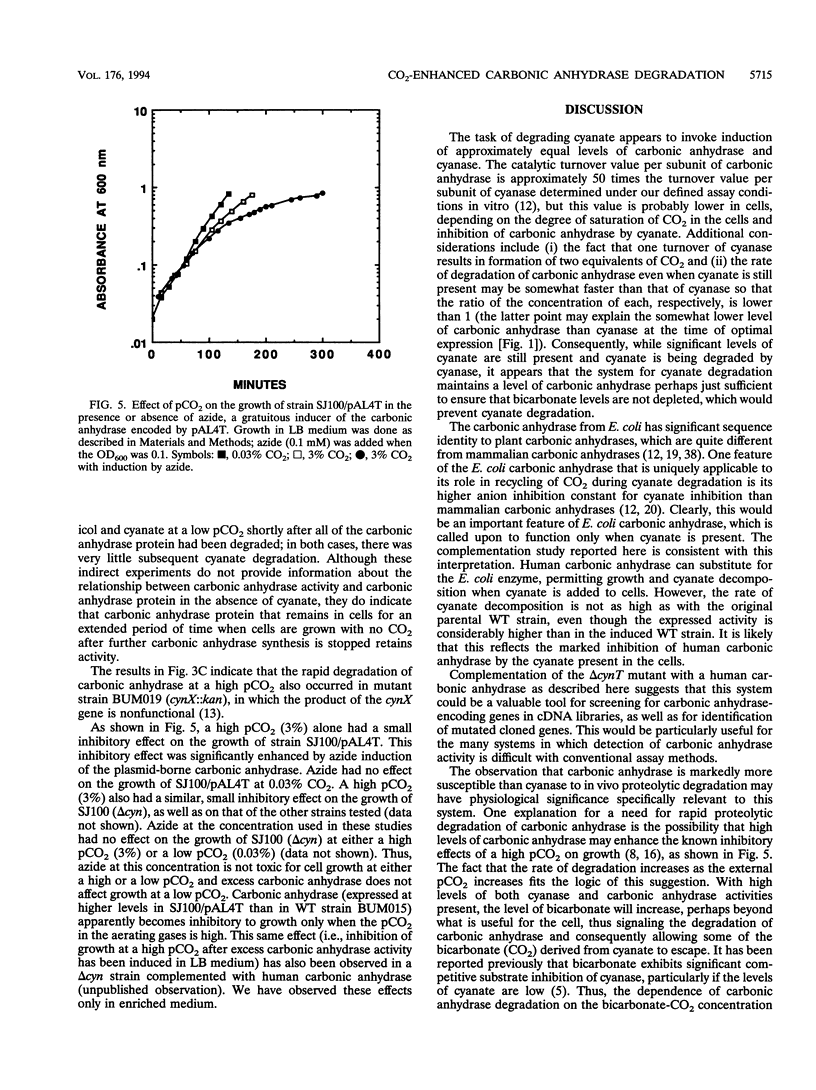
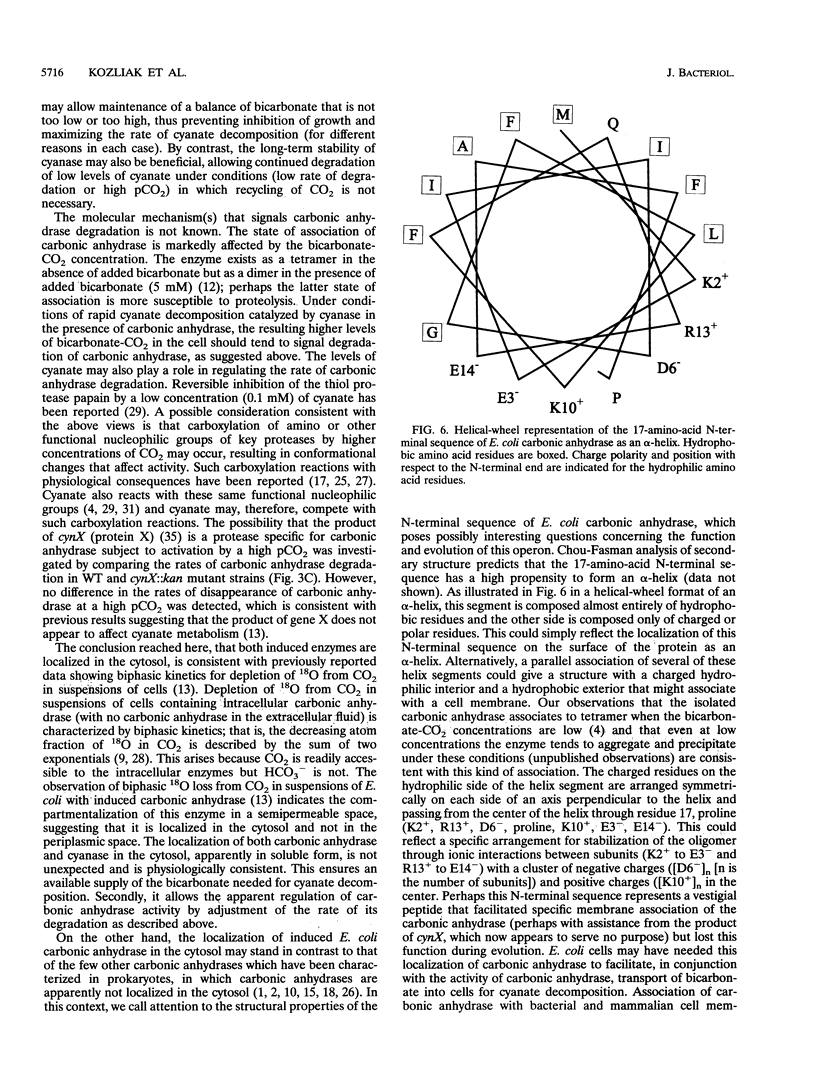
Cyanase catalyzes the reaction of cyanate with bicarbonate to give 2CO2. The cynS gene encoding cyanase, together with the cynT gene for carbonic anhydrase, is part of the cyn operon, the expression of which is induced in Escherichia coli by cyanate. The physiological role of carbonic anhydrase is to prevent depletion of cellular bicarbonate during cyanate decomposition due to loss of CO2 (M.B. Guilloton, A.F. Lamblin, E. I. Kozliak, M. Gerami-Nejad, C. Tu, D. Silverman, P.M. Anderson, and J.A. Fuchs, J. Bacteriol. 175:1443-1451, 1993). A delta cynT mutant strain was extremely sensitive to inhibition of growth by cyanate and did not catalyze decomposition of cyanate (even though an active cyanase was expressed) when grown at a low pCO2 (in air) but had a Cyn+ phenotype at a high pCO2. Here the expression of these two enzymes in this unusual system for cyanate degradation was characterized in more detail. Both enzymes were found to be located in the cytosol and to be present at approximately equal levels in the presence of cyanate. A delta cynT mutant strain could be complemented with high levels of expressed human carbonic anhydrase II; however, the mutant defect was not completely abolished, perhaps because the E. coli carbonic anhydrase is significantly less susceptible to inhibition by cyanate than mammalian carbonic anhydrases. The induced E. coli carbonic anhydrase appears to be particularly adapted to its function in cyanate degradation. Active cyanase remained in cells grown in the presence of either low or high pCO2 after the inducer cyanate was depleted; in contrast, carbonic anhydrase protein was degraded very rapidly (minutes) at a high pCO2 but much more slowly (hours) at a low pCO2. A physiological significance of these observations is suggested by the observation that expression of carbonic anhydrase at a high pCO2 decreased the growth rate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler L., Brundell J., Falkbring S. O., Nyman P. O. Carbonic anhydrase from Neisseria sicca, strain 6021. I. Bacterial growth and purification of the enzyme. Biochim Biophys Acta. 1972 Sep 19;284(1):298–310. doi: 10.1016/0005-2744(72)90068-x. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Carlson J. D. Reversible reaction of cyanate with a reactive sulfhydryl group at the glutamine binding site of carbamyl phosphate synthetase. Biochemistry. 1975 Aug 12;14(16):3688–3694. doi: 10.1021/bi00687a027. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Johnson W. V., Endrizzi J. A., Little R. M., Korte J. J. Interaction of mono- and dianions with cyanase: evidence for apparent half-site binding. Biochemistry. 1987 Jun 30;26(13):3938–3943. doi: 10.1021/bi00387a029. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Little R. M. Kinetic properties of cyanase. Biochemistry. 1986 Apr 8;25(7):1621–1626. doi: 10.1021/bi00355a026. [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Purification and properties of the inducible enzyme cyanase. Biochemistry. 1980 Jun 24;19(13):2882–2888. doi: 10.1021/bi00554a010. [DOI] [PubMed] [Google Scholar]
- Diaz E., Sandblom J. P., Wistrand P. J. Selectivity properties of channels induced by a reconstituted membrane-bound carbonic anhydrase. Acta Physiol Scand. 1982 Dec;116(4):461–463. doi: 10.1111/j.1748-1716.1982.tb07167.x. [DOI] [PubMed] [Google Scholar]
- Dixon N. M., Kell D. B. The inhibition by CO2 of the growth and metabolism of micro-organisms. J Appl Bacteriol. 1989 Aug;67(2):109–136. doi: 10.1111/j.1365-2672.1989.tb03387.x. [DOI] [PubMed] [Google Scholar]
- Forster R. E., 2nd, Dodgson S. J., Storey B. T., Lin L. Measurement of carbonic anhydrase activity inside cells and subcellular particles. Ann N Y Acad Sci. 1984;429:415–429. doi: 10.1111/j.1749-6632.1984.tb12368.x. [DOI] [PubMed] [Google Scholar]
- Fukuzawa H., Suzuki E., Komukai Y., Miyachi S. A gene homologous to chloroplast carbonic anhydrase (icfA) is essential to photosynthetic carbon dioxide fixation by Synechococcus PCC7942. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4437–4441. doi: 10.1073/pnas.89.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloton M. B., Korte J. J., Lamblin A. F., Fuchs J. A., Anderson P. M. Carbonic anhydrase in Escherichia coli. A product of the cyn operon. J Biol Chem. 1992 Feb 25;267(6):3731–3734. [PubMed] [Google Scholar]
- Guilloton M. B., Lamblin A. F., Kozliak E. I., Gerami-Nejad M., Tu C., Silverman D., Anderson P. M., Fuchs J. A. A physiological role for cyanate-induced carbonic anhydrase in Escherichia coli. J Bacteriol. 1993 Mar;175(5):1443–1451. doi: 10.1128/jb.175.5.1443-1451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloton M., Karst F. Isolation and characterization of Escherichia coli mutants lacking inducible cyanase. J Gen Microbiol. 1987 Mar;133(3):645–653. doi: 10.1099/00221287-133-3-645. [DOI] [PubMed] [Google Scholar]
- MacLeod M. N., DeVoe I. W. Localization of carbonic anhydrase in the cytoplasmic membrane of Neisseria sicca (strain 19). Can J Microbiol. 1981 Jan;27(1):87–92. doi: 10.1139/m81-014. [DOI] [PubMed] [Google Scholar]
- Majeau N., Coleman J. R. Isolation and characterization of a cDNA coding for pea chloroplastic carbonic anhydrase. Plant Physiol. 1991 Jan;95(1):264–268. doi: 10.1104/pp.95.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H., Sanyal G. The activity of sulfonamides and anions against the carbonic anhydrases of animals, plants, and bacteria. Annu Rev Pharmacol Toxicol. 1983;23:439–459. doi: 10.1146/annurev.pa.23.040183.002255. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. J., Alston T. A., Bright H. J. CO2 adducts as reactive analogues of carboxylate substrates for aconitase and other enzymes of carbohydrate metabolism. J Biol Chem. 1987 May 15;262(14):6552–6563. [PubMed] [Google Scholar]
- Price G. D., Coleman J. R., Badger M. R. Association of Carbonic Anhydrase Activity with Carboxysomes Isolated from the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1992 Oct;100(2):784–793. doi: 10.1104/pp.100.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein J. E., Parsons S. M. Origin of the bicarbonate stimulation of Torpedo electric organ synaptic vesicle ATPase. J Neurochem. 1982 Dec;39(6):1660–1668. doi: 10.1111/j.1471-4159.1982.tb08000.x. [DOI] [PubMed] [Google Scholar]
- Silverman D. N., Tu C. K., Roessler N. Diffusion-limited exchange of 18O between CO2 and water in red cell suspensions. Respir Physiol. 1981 Jun;44(3):285–298. doi: 10.1016/0034-5687(81)90024-4. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A. Reversible inactivation of papain by cyanate. Biochim Biophys Acta. 1967 Jul 11;139(2):439–449. doi: 10.1016/0005-2744(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sung Y. C., Anderson P. M., Fuchs J. A. Characterization of high-level expression and sequencing of the Escherichia coli K-12 cynS gene encoding cyanase. J Bacteriol. 1987 Nov;169(11):5224–5230. doi: 10.1128/jb.169.11.5224-5230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y. C., Fuchs J. A. Characterization of the cyn operon in Escherichia coli K12. J Biol Chem. 1988 Oct 15;263(29):14769–14775. [PubMed] [Google Scholar]
- Sung Y. C., Parsell D., Anderson P. M., Fuchs J. A. Identification, mapping, and cloning of the gene encoding cyanase in Escherichia coli K-12. J Bacteriol. 1987 Jun;169(6):2639–2642. doi: 10.1128/jb.169.6.2639-2642.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhauser S. M., Jewell D. A., Tu C. K., Silverman D. N., Laipis P. J. A T7 expression vector optimized for site-directed mutagenesis using oligodeoxyribonucleotide cassettes. Gene. 1992 Aug 1;117(1):113–117. doi: 10.1016/0378-1119(92)90498-e. [DOI] [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]