Abstract
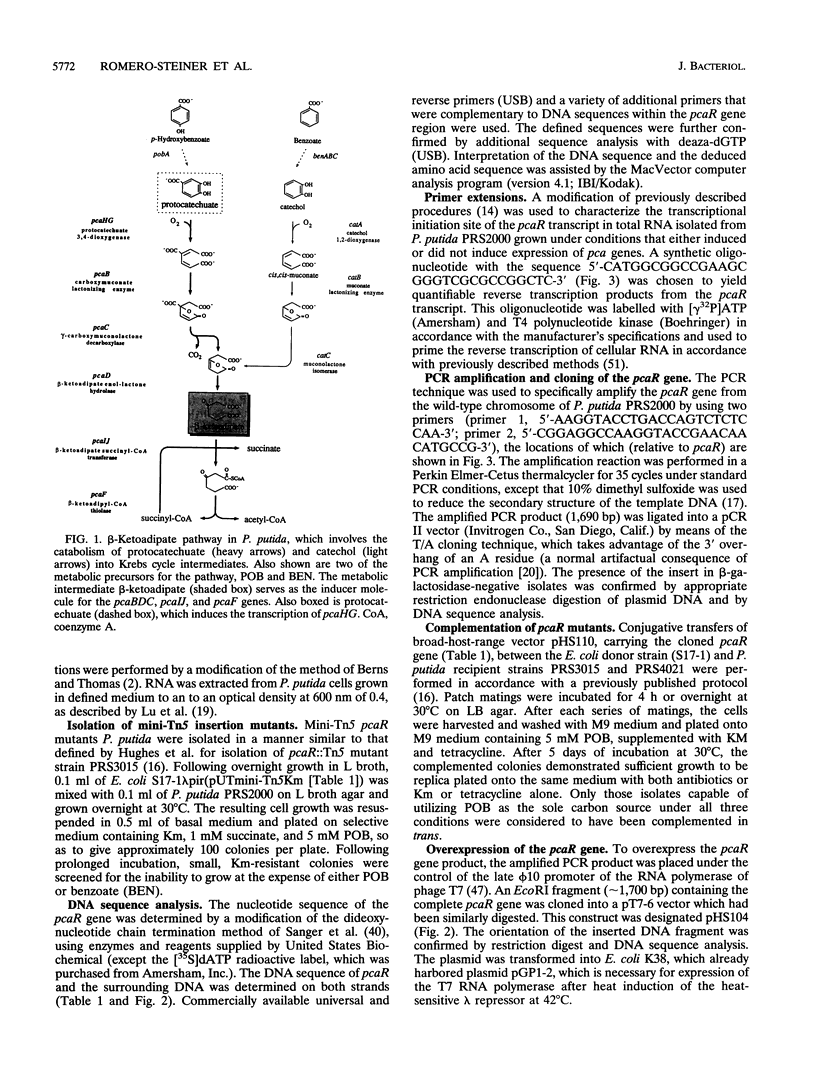
The pca branch of the beta-ketoadipate pathway in Pseudomonas putida is responsible for the complete degradation of p-hydroxybenzoate through ortho cleavage of the initial pathway metabolite, protocatechuate. The pcaR regulatory locus has been found to be required for both induction of all of the genes within the pca regulon (pcaBDC, pcaIJ, and pcaF) and the chemotactic response of the bacteria to aromatic compounds. Insertional inactivation mutagenesis, using Tn5 and mini-Tn5 transposons, was used to locate, clone, and sequence this pcaR regulatory gene. The pcaR gene product, when overexpressed in Escherichia coli, possessed a specific affinity for the pcaIJ promoter region and demonstrated that the entire PcaR protein was required for this function. The deduced amino acid sequence of the PcaR regulatory peptide bears little resemblance to its counterpart in the other branch of the pathway, CatR, but exhibits significant homology to its regulatory antecedent, PobR, which regulates the initial breakdown of p-hydroxybenzoate into protocatechuate. Comparisons of the pcaIJ and pcaR promoter regions revealed conservation of a 15-bp sequence centered around the -10 region in both sequences. This, together with previously defined deletional studies with the pcaIJ promoter region, suggests that PcaR exerts its regulatory effect through protein-DNA interactions within this region, which would be unusually close to the transcriptional start site of pcaIJ for a positive regulator.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Z., Chael M. L., O'Halloran T. V. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature. 1992 Jan 2;355(6355):87–89. doi: 10.1038/355087a0. [DOI] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Coco W. M., Rothmel R. K., Henikoff S., Chakrabarty A. M. Nucleotide sequence and initial functional characterization of the clcR gene encoding a LysR family activator of the clcABD chlorocatechol operon in Pseudomonas putida. J Bacteriol. 1993 Jan;175(2):417–427. doi: 10.1128/jb.175.2.417-427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco A. A., Averhoff B., Ornston L. N. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J Bacteriol. 1993 Jul;175(14):4499–4506. doi: 10.1128/jb.175.14.4499-4506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazee R. W., Livingston D. M., LaPorte D. C., Lipscomb J. D. Cloning, sequencing, and expression of the Pseudomonas putida protocatechuate 3,4-dioxygenase genes. J Bacteriol. 1993 Oct;175(19):6194–6202. doi: 10.1128/jb.175.19.6194-6202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. G., Gussin G. N. RNA polymerases from Pseudomonas aeruginosa and Pseudomonas syringae respond to Escherichia coli activator proteins. J Bacteriol. 1991 Jan;173(1):394–397. doi: 10.1128/jb.173.1.394-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C. Y., Crawford I. P., Harwood C. S. Up-promoter mutations in the trpBA operon of Pseudomonas aeruginosa. J Bacteriol. 1991 Jun;173(12):3756–3762. doi: 10.1128/jb.173.12.3756-3762.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Rivelli M., Ornston L. N. Aromatic acids are chemoattractants for Pseudomonas putida. J Bacteriol. 1984 Nov;160(2):622–628. doi: 10.1128/jb.160.2.622-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K. Regulation of synthesis of early enzymes of p-hydroxybenzoate pathway in Pseudomonas putida. J Biol Chem. 1970 Oct 25;245(20):5304–5308. [PubMed] [Google Scholar]
- Hughes E. J., Shapiro M. K., Houghton J. E., Ornston L. N. Cloning and expression of pca genes from Pseudomonas putida in Escherichia coli. J Gen Microbiol. 1988 Nov;134(11):2877–2887. doi: 10.1099/00221287-134-11-2877. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Lu C. D., Kilstrup M., Neuhard J., Abdelal A. Pyrimidine regulation of tandem promoters for carAB in Salmonella typhimurium. J Bacteriol. 1989 Oct;171(10):5436–5442. doi: 10.1128/jb.171.10.5436-5442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Pey N. K., Herrnstadt C., Marcil R. A., Smith L. M. A universal method for the direct cloning of PCR amplified nucleic acid. Biotechnology (N Y) 1991 Jul;9(7):657–663. doi: 10.1038/nbt0791-657. [DOI] [PubMed] [Google Scholar]
- Nègre D., Cortay J. C., Old I. G., Galinier A., Richaud C., Saint Girons I., Cozzone A. J. Overproduction and characterization of the iclR gene product of Escherichia coli K-12 and comparison with that of Salmonella typhimurium LT2. Gene. 1991 Jan 2;97(1):29–37. doi: 10.1016/0378-1119(91)90006-w. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell. 1989 Jan 13;56(1):119–129. doi: 10.1016/0092-8674(89)90990-2. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Evolution of catabolic pathways. Biochem Soc Trans. 1976;4(3):468–472. doi: 10.1042/bst0040468. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Properties of an inducible uptake system for beta-ketoadipate in Pseudomonas putida. J Bacteriol. 1976 Feb;125(2):475–488. doi: 10.1128/jb.125.2.475-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. The evolution of induction mechanisms in bacteria: insights derived from the study of the beta-ketoadipate pathway. Curr Top Cell Regul. 1977;12:209–262. doi: 10.1016/b978-0-12-152812-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Parales R. E., Harwood C. S. Regulation of the pcaIJ genes for aromatic acid degradation in Pseudomonas putida. J Bacteriol. 1993 Sep;175(18):5829–5838. doi: 10.1128/jb.175.18.5829-5838.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsek M. R., Shinabarger D. L., Rothmel R. K., Chakrabarty A. M. Roles of CatR and cis,cis-muconate in activation of the catBC operon, which is involved in benzoate degradation in Pseudomonas putida. J Bacteriol. 1992 Dec;174(23):7798–7806. doi: 10.1128/jb.174.23.7798-7806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Rothmel R. K., Aldrich T. L., Houghton J. E., Coco W. M., Ornston L. N., Chakrabarty A. M. Nucleotide sequencing and characterization of Pseudomonas putida catR: a positive regulator of the catBC operon is a member of the LysR family. J Bacteriol. 1990 Feb;172(2):922–931. doi: 10.1128/jb.172.2.922-931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothmel R. K., Shinabarger D. L., Parsek M. R., Aldrich T. L., Chakrabarty A. M. Functional analysis of the Pseudomonas putida regulatory protein CatR: transcriptional studies and determination of the CatR DNA-binding site by hydroxyl-radical footprinting. J Bacteriol. 1991 Aug;173(15):4717–4724. doi: 10.1128/jb.173.15.4717-4724.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Replacement of the fip gene of Escherichia coli by an inactive gene cloned on a plasmid. J Bacteriol. 1984 Sep;159(3):1034–1039. doi: 10.1128/jb.159.3.1034-1039.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Smith C. P., Chater K. F. Structure and regulation of controlling sequences for the Streptomyces coelicolor glycerol operon. J Mol Biol. 1988 Dec 5;204(3):569–580. doi: 10.1016/0022-2836(88)90356-7. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Sunnarborg A., Klumpp D., Chung T., LaPorte D. C. Regulation of the glyoxylate bypass operon: cloning and characterization of iclR. J Bacteriol. 1990 May;172(5):2642–2649. doi: 10.1128/jb.172.5.2642-2649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- West S. E., Iglewski B. H. Codon usage in Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Oct 11;16(19):9323–9335. doi: 10.1093/nar/16.19.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. E., Woolridge E. M., Ransom S. C., Landro J. A., Babbitt P. C., Kozarich J. W. 3-Carboxy-cis,cis-muconate lactonizing enzyme from Pseudomonas putida is homologous to the class II fumarase family: a new reaction in the evolution of a mechanistic motif. Biochemistry. 1992 Oct 13;31(40):9768–9776. doi: 10.1021/bi00155a033. [DOI] [PubMed] [Google Scholar]
- Wong S. C., Abdelal A. T. Unorthodox expression of an enzyme: evidence for an untranslated region within carA from Pseudomonas aeruginosa. J Bacteriol. 1990 Feb;172(2):630–642. doi: 10.1128/jb.172.2.630-642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Evolutionarily homologous alpha 2 beta 2 oligomeric structures in beta-ketoadipate succinyl-CoA transferases from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1981 Feb 25;256(4):1565–1569. [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990 Nov;172(11):6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]