Abstract
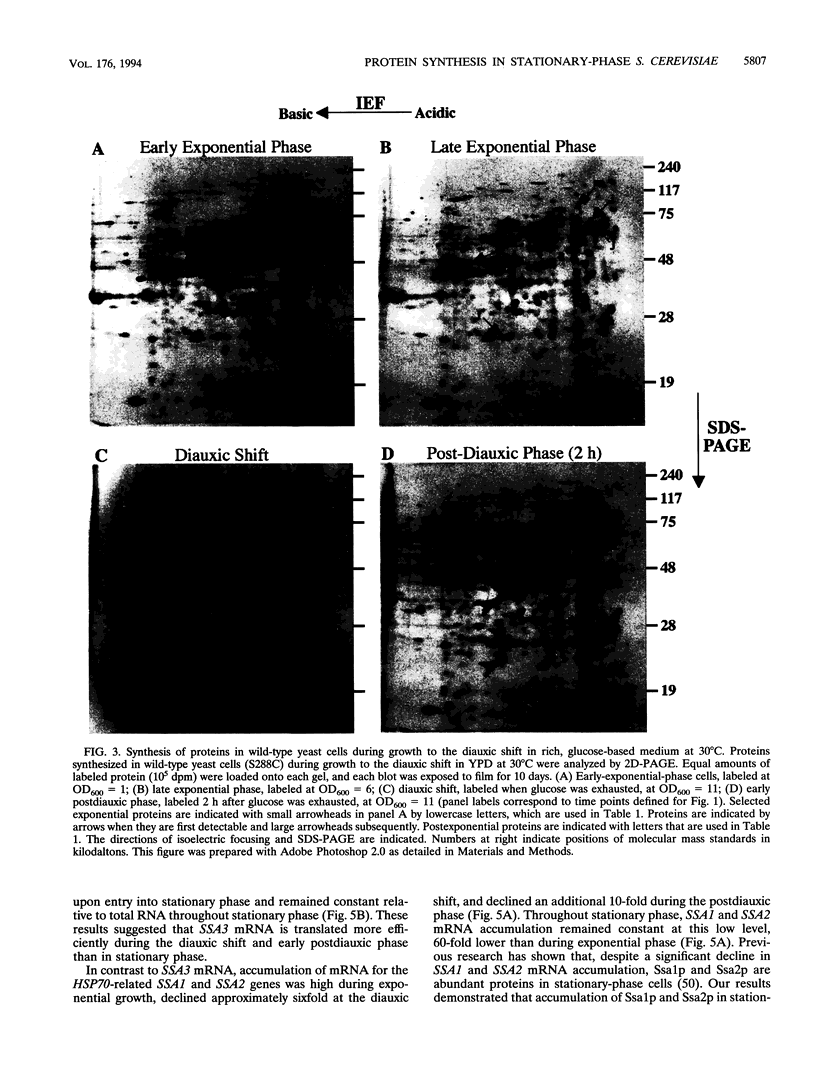
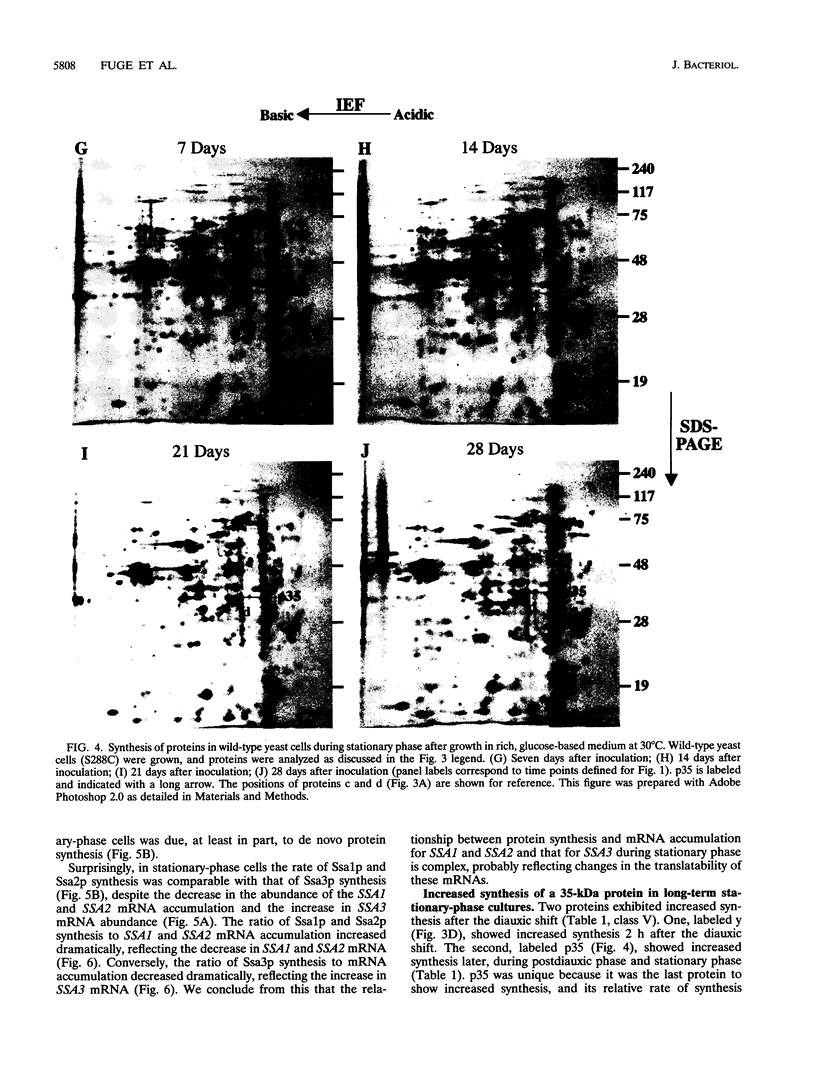
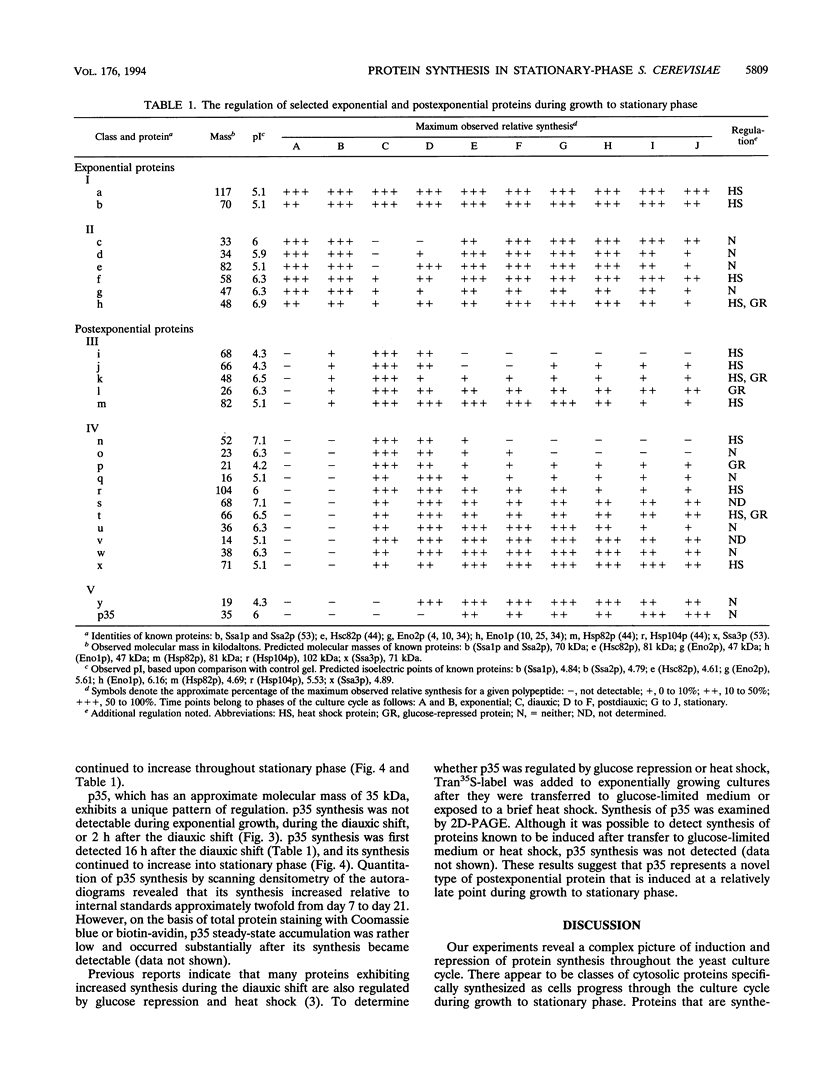
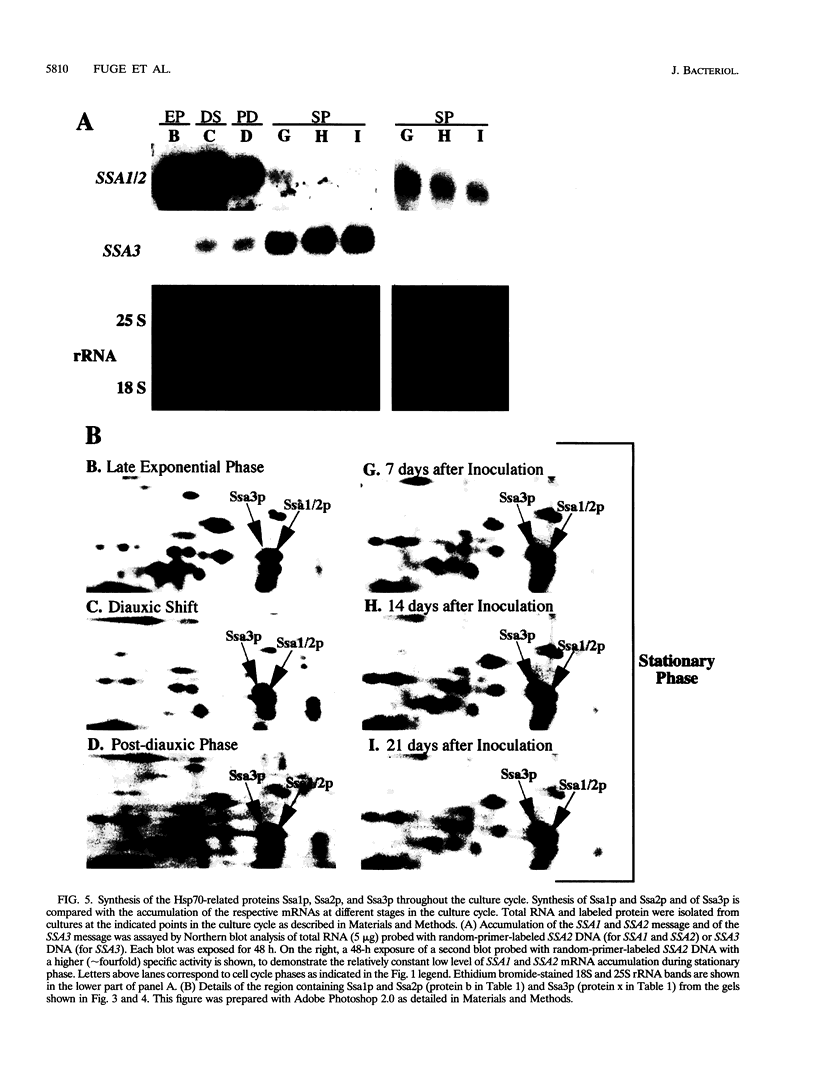
We are interested in characterizing the process of entry into and the maintenance of the stationary phase. To identify proteins that are induced during growth to stationary phase, we examined protein synthesis in long-term stationary-phase cultures using two-dimensional polyacrylamide gel electrophoresis (2D-PAGE). Although the total rate of protein synthesis declined when growth ceased after the postdiauxic phase, the pattern of proteins synthesized remained similar throughout the experimental period (28 days), except at the diauxic shift. At the diauxic shift most proteins detectable by 2D-PAGE undergo a transient reduction in their relative rate of synthesis that ends when cells resume growth during the postdiauxic phase. We conclude from this that the transient repression of protein synthesis at the diauxic shift is not directly associated with stationary-phase arrest. A number of proteins that are synthesized after exponential phase have been identified by 2D-PAGE. These proteins could be divided into three temporal classes depending upon when their synthesis became detectable. One postexponential protein, designated p35, was induced later than all other proteins, and its relative rate of synthesis increased throughout stationary phase. Unlike most postexponential proteins, p35 was not regulated by heat shock or glucose repression. We also observed that a direct correlation between steady-state mRNA accumulation and protein synthesis for another postexponential protein (Ssa3p) or two closely related constitutive proteins (Ssa1p and Ssa2p) did not exist. We conclude from this result that synthesis of proteins in stationary phase is regulated by mechanisms other than the control of steady-state mRNA accumulation.
Full text
PDF


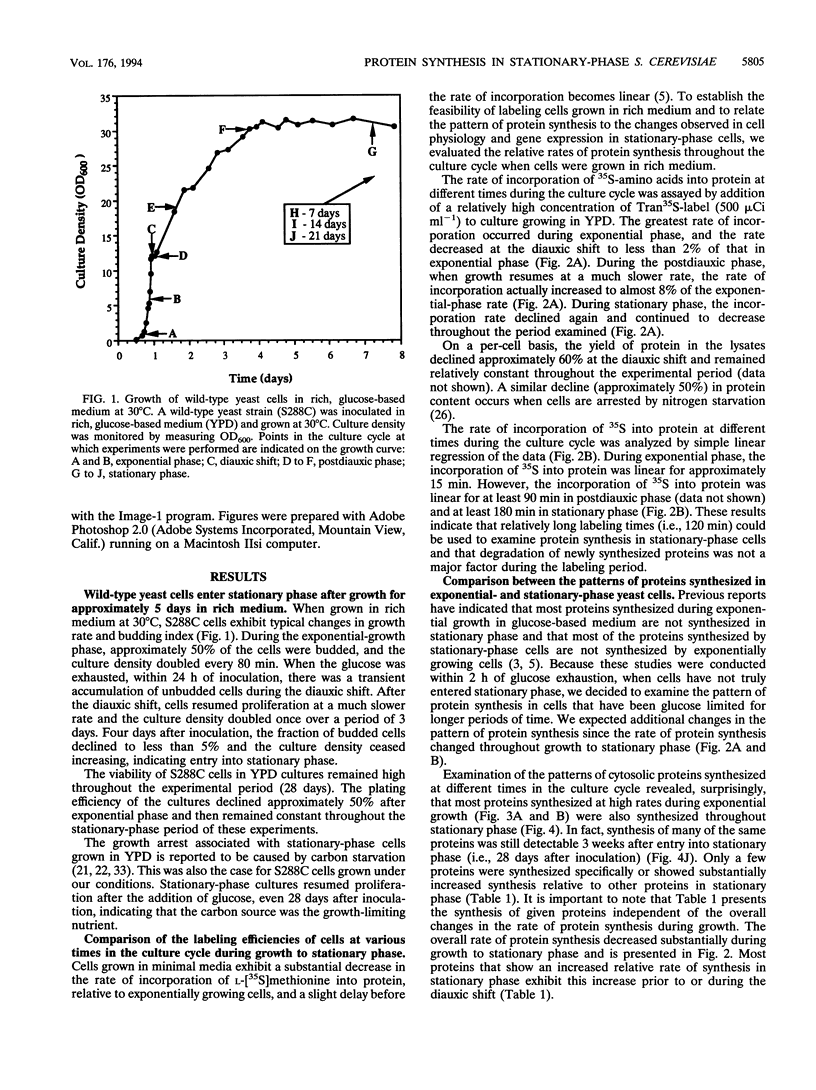
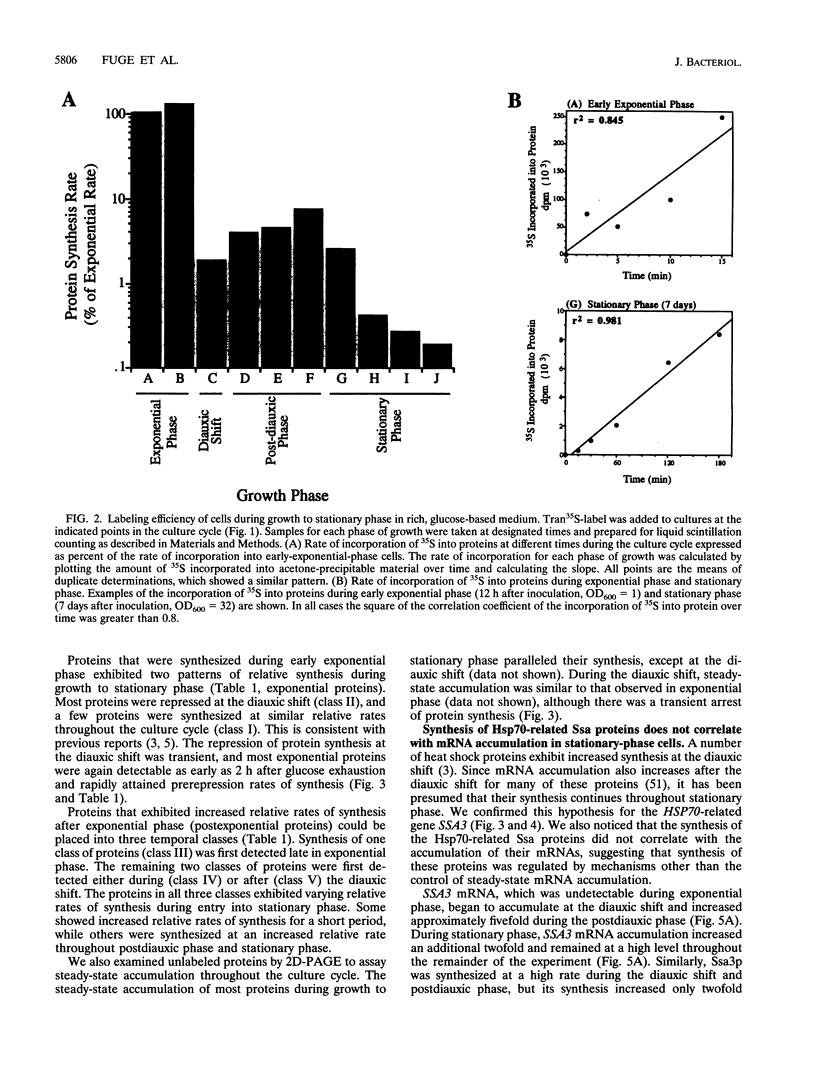


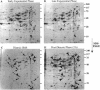
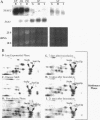
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almirón M., Link A. J., Furlong D., Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992 Dec;6(12B):2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- Bataillé N., Régnacq M., Boucherie H. Induction of a heat-shock-type response in Saccharomyces cerevisiae following glucose limitation. Yeast. 1991 May-Jun;7(4):367–378. doi: 10.1002/yea.320070407. [DOI] [PubMed] [Google Scholar]
- Bataillé N., Thoraval D., Boucherie H. Two-dimensional gel analysis of yeast proteins: application to the study of changes in the levels of major polypeptides of Saccharomyces cerevisiae depending on the fermentable or nonfermentable nature of the carbon source. Electrophoresis. 1988 Nov;9(11):774–780. doi: 10.1002/elps.1150091113. [DOI] [PubMed] [Google Scholar]
- Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985 Jan;161(1):385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario D., Hinnebusch A. G., Dhar R. Multiple regulatory mechanisms control the expression of the RAS1 and RAS2 genes of Saccharomyces cerevisiae. EMBO J. 1988 Jun;7(6):1805–1813. doi: 10.1002/j.1460-2075.1988.tb03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario D., Hinnebusch A., Cannon J., Tatchell K., Dhar R. Carbon source regulation of RAS1 expression in Saccharomyces cerevisiae and the phenotypes of ras2- cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4152–4156. doi: 10.1073/pnas.83.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R. RAS genes in Saccharomyces cerevisiae: signal transduction in search of a pathway. Trends Genet. 1991 Jan;7(1):28–33. doi: 10.1016/0168-9525(91)90018-l. [DOI] [PubMed] [Google Scholar]
- Brousse M., Bataillé N., Boucherie H. Identification of Glycolytic Enzyme Polypeptides on the Two-Dimensional Protein Map of Saccharomyces cerevisiae and Application to the Study of Some Wine Yeasts. Appl Environ Microbiol. 1985 Oct;50(4):951–957. doi: 10.1128/aem.50.4.951-957.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Carlson M., Osmond B. C., Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981 May;98(1):25–40. doi: 10.1093/genetics/98.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M. A general topoisomerase I-dependent transcriptional repression in the stationary phase in yeast. Genes Dev. 1991 Dec;5(12A):2315–2326. doi: 10.1101/gad.5.12a.2315. [DOI] [PubMed] [Google Scholar]
- Choder M. A growth rate-limiting process in the last growth phase of the yeast life cycle involves RPB4, a subunit of RNA polymerase II. J Bacteriol. 1993 Oct;175(19):6358–6363. doi: 10.1128/jb.175.19.6358-6363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy R. G., Pardee A. B. Enhanced synthesis and stabilization of Mr 68,000 protein in transformed BALB/c-3T3 cells: candidate for restriction point control of cell growth. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4699–4703. doi: 10.1073/pnas.80.15.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebot M. A., Barnes C. A., Singer R. A., Johnston G. C. Genetic assessment of stationary phase for cells of the yeast Saccharomyces cerevisiae. J Bacteriol. 1990 Jul;172(7):3584–3589. doi: 10.1128/jb.172.7.3584-3589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebot M. A., Johnston G. C., Singer R. A. A yeast mutant conditionally defective only for reentry into the mitotic cell cycle from stationary phase. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7948–7952. doi: 10.1073/pnas.84.22.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B., Futcher B. Stress resistance of yeast cells is largely independent of cell cycle phase. Yeast. 1993 Jan;9(1):33–42. doi: 10.1002/yea.320090105. [DOI] [PubMed] [Google Scholar]
- Gates B. J., Friedkin M. Mid-G1 marker protein(s) in 3T3 mouse fibroblast cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4959–4961. doi: 10.1073/pnas.75.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot D., Snyder M. Carbon source induces growth of stationary phase yeast cells, independent of carbon source metabolism. Yeast. 1993 May;9(5):465–479. doi: 10.1002/yea.320090503. [DOI] [PubMed] [Google Scholar]
- Granot D., Snyder M. Glucose induces cAMP-independent growth-related changes in stationary-phase cells of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5724–5728. doi: 10.1073/pnas.88.13.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Iida H., Yahara I. Durable synthesis of high molecular weight heat shock proteins in G0 cells of the yeast and other eucaryotes. J Cell Biol. 1984 Jul;99(1 Pt 1):199–207. doi: 10.1083/jcb.99.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A., McFarlane S. Growth and cell division during nitrogen starvation of the yeast Saccharomyces cerevisiae. J Bacteriol. 1977 Nov;132(2):723–730. doi: 10.1128/jb.132.2.723-730.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:428–453. doi: 10.1016/0076-6879(91)94034-a. [DOI] [PubMed] [Google Scholar]
- Kolter R., Siegele D. A., Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- Köhrer K., Domdey H. Preparation of high molecular weight RNA. Methods Enzymol. 1991;194:398–405. doi: 10.1016/0076-6879(91)94030-g. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagunas R. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast. 1986 Dec;2(4):221–228. doi: 10.1002/yea.320020403. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Foy J. J., Elliott S. G., McLaughlin C. S. Synthesis of specific identified, phosphorylated, heat shock, and heat stroke proteins through the cell cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):117–126. doi: 10.1128/mcb.2.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Johnston J. R. Genealogy of principal strains of the yeast genetic stock center. Genetics. 1986 May;113(1):35–43. doi: 10.1093/genetics/113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet C. M., Craig E. A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989 Sep;9(9):3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Riddle V. G., Dubrow R., Pardee A. B. Changes in the synthesis of actin and other cell proteins after stimulation of serum-arrested cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1298–1302. doi: 10.1073/pnas.76.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley A., Johnston G. C., Butler B., Werner-Washburne M., Singer R. A. Heat shock-mediated cell cycle blockage and G1 cyclin expression in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1993 Feb;13(2):1034–1041. doi: 10.1128/mcb.13.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M., Bradshaw-Rouse J., Markwardt D., Heideman W. Changes in gene expression in the Ras/adenylate cyclase system of Saccharomyces cerevisiae: correlation with cAMP levels and growth arrest. Mol Biol Cell. 1993 Jul;4(7):757–765. doi: 10.1091/mbc.4.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Parsell D. A., Taulien J., Vogel J. L., Craig E. A., Lindquist S. Genetic evidence for a functional relationship between Hsp104 and Hsp70. J Bacteriol. 1993 Oct;175(20):6484–6491. doi: 10.1128/jb.175.20.6484-6491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992 Oct;119(2):301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Iida H., Pardee A. B. Identification of a novel stress-inducible glycoprotein in Saccharomyces cerevisiae. I. Preliminary characterization. J Biol Chem. 1988 Jun 25;263(18):8569–8575. [PubMed] [Google Scholar]
- Verma R., Iida H., Pardee A. B. Modulation of expression of the stress-inducible p118 of Saccharomyces cerevisiae by cAMP. II. A study of p118 expression in mutants of the cAMP cascade. J Biol Chem. 1988 Jun 25;263(18):8576–8582. [PubMed] [Google Scholar]
- Werner-Washburne M., Becker J., Kosic-Smithers J., Craig E. A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Braun E., Johnston G. C., Singer R. A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993 Jun;57(2):383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Brown D., Braun E. Bcy1, the regulatory subunit of cAMP-dependent protein kinase in yeast, is differentially modified in response to the physiological status of the cell. J Biol Chem. 1991 Oct 15;266(29):19704–19709. [PubMed] [Google Scholar]
- Werner-Washburne M., Stone D. E., Craig E. A. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jul;7(7):2568–2577. doi: 10.1128/mcb.7.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik N. A., Young R. A. RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol Cell Biol. 1989 Jul;9(7):2854–2859. doi: 10.1128/mcb.9.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]