Abstract
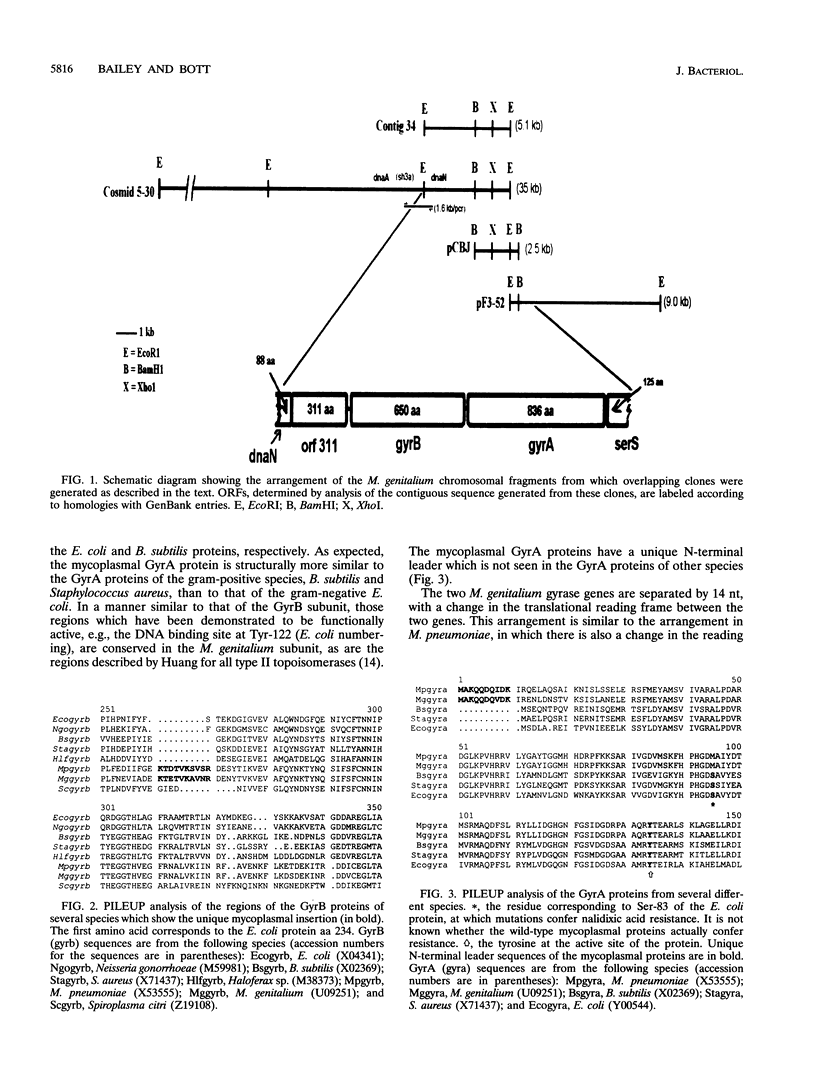
Origins of replication are known to be highly conserved among widely divergent microbial species, with the gene order in those regions being dnaA-dnaN-recF-gyrB. On the basis of sequence identities to entries in GenBank, the gene order of a 6-kb fragment of Mycoplasma genitalium DNA was determined to be dnaN-orf311-gyrB-gyrA-serS, which is structurally similar to the ancestral origin of replication. We have directly linked the dnaN gene to the M. genitalium dnaA gene by PCR amplification. However, we found a novel open reading frame, designated orf311, in place of an expected sequence encoding recF. Orf311 contains a DnaJ box motif at its N terminus, but it has no overall homology to any other protein or sequence in the database. We are unable to detect any recF homolog in M. genitalium by hybridization or during a random sequencing survey of the genome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C., Lüder G., Tailor R. H. Characterization of Bacillus subtilis recombinational pathways. J Bacteriol. 1991 Jul;173(13):3977–3980. doi: 10.1128/jb.173.13.3977-3980.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Sander C., Valencia A., Bukau B. A module of the DnaJ heat shock proteins found in malaria parasites. Trends Biochem Sci. 1992 Apr;17(4):129–129. doi: 10.1016/0968-0004(92)90319-5. [DOI] [PubMed] [Google Scholar]
- Brockbank S. M., Barth P. T. Cloning, sequencing, and expression of the DNA gyrase genes from Staphylococcus aureus. J Bacteriol. 1993 Jun;175(11):3269–3277. doi: 10.1128/jb.175.11.3269-3277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol Biol Cell. 1993 Jun;4(6):555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Bott K. F. Mycoplasma pneumoniae DNA gyrase genes. Mol Microbiol. 1990 Jul;4(7):1129–1134. doi: 10.1111/j.1365-2958.1990.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Litaker W., Bott K. F. A physical map of the Mycoplasma genitalium genome. Mol Microbiol. 1990 Apr;4(4):683–687. doi: 10.1111/j.1365-2958.1990.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Davies C. J., Hutchison C. A., 3rd A directed DNA sequencing strategy based upon Tn3 transposon mutagenesis: application to the ADE1 locus on Saccharomyces cerevisiae chromosome I. Nucleic Acids Res. 1991 Oct 25;19(20):5731–5738. doi: 10.1093/nar/19.20.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Hollingshead S. K., Heath D. G., Clewell D. B., Sun F., Woodard A. Degenerate oligonucleotide primers for enzymatic amplification of recA sequences from gram-positive bacteria and mycoplasmas. J Bacteriol. 1992 Apr;174(8):2729–2732. doi: 10.1128/jb.174.8.2729-2732.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. Y., Baumann P. Genetic analysis of an aphid endosymbiont DNA fragment homologous to the rnpA-rpmH-dnaA-dnaN-gyrB region of eubacteria. Gene. 1992 Apr 15;113(2):175–181. doi: 10.1016/0378-1119(92)90393-4. [DOI] [PubMed] [Google Scholar]
- Lampe M. F., Bott K. F. Genetic and physical organization of the cloned gyrA and gyrB genes of Bacillus subtilis. J Bacteriol. 1985 Apr;162(1):78–84. doi: 10.1128/jb.162.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju M. V., Clark A. J. Evidence for ATP binding and double-stranded DNA binding by Escherichia coli RecF protein. J Bacteriol. 1992 Dec;174(23):7705–7710. doi: 10.1128/jb.174.23.7705-7710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M., Sano K., Okada R., Fukumura T. Mapping of replication initiation site in Mycoplasma capricolum genome by two-dimensional gel-electrophoretic analysis. Nucleic Acids Res. 1993 Oct 11;21(20):4816–4823. doi: 10.1093/nar/21.20.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Yoshikawa H. Genes and their organization in the replication origin region of the bacterial chromosome. Mol Microbiol. 1992 Mar;6(5):629–634. doi: 10.1111/j.1365-2958.1992.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Old I. G., Margarita D., Saint Girons I. Unique genetic arrangement in the dnaA region of the Borrelia burgdorferi linear chromosome: nucleotide sequence of the dnaA gene. FEMS Microbiol Lett. 1993 Jul 15;111(1):109–114. doi: 10.1111/j.1574-6968.1993.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Peterson S. N., Hu P. C., Bott K. F., Hutchison C. A., 3rd A survey of the Mycoplasma genitalium genome by using random sequencing. J Bacteriol. 1993 Dec;175(24):7918–7930. doi: 10.1128/jb.175.24.7918-7930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S. N., Schramm N., Hu P. C., Bott K. F., Hutchison C. A., 3rd A random sequencing approach for placing markers on the physical map of Mycoplasma genitalium. Nucleic Acids Res. 1991 Nov 11;19(21):6027–6031. doi: 10.1093/nar/19.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Molecular biology and genetics of mycoplasmas (Mollicutes). Microbiol Rev. 1985 Dec;49(4):419–455. doi: 10.1128/mr.49.4.419-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Yee T. W., Baird C., Krishnapillai V. Pseudomonad replication origins: a paradigm for bacterial origins? Mol Microbiol. 1991 Nov;5(11):2581–2587. doi: 10.1111/j.1365-2958.1991.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tamura J. K., Gellert M. Characterization of the ATP binding site on Escherichia coli DNA gyrase. Affinity labeling of Lys-103 and Lys-110 of the B subunit by pyridoxal 5'-diphospho-5'-adenosine. J Biol Chem. 1990 Dec 5;265(34):21342–21349. [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweiger G., Shapiro L. Expression of Caulobacter dnaA as a function of the cell cycle. J Bacteriol. 1994 Jan;176(2):401–408. doi: 10.1128/jb.176.2.401-408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]



