Abstract
Results on the subcellular localization of the mineralocorticoid receptor (MR) have been controversial. To determine the subcellular distribution and trafficking of the MR in living cells after binding of agonists and antagonists, we expressed a MR-green fluorescent protein (GFP) chimera in mammalian cells lacking endogenous MR. The GFP-tagged MR (GFP-MR) remained transcriptionally active, as determined in cotransfection experiments with the MR-responsive reporter, TAT3-LUC. The subcellular localization of GFP-MR was monitored by fluorescence time-lapse microscopy. In the absence of hormone, MR was present both in the cytoplasm and nucleus. Aldosterone induced a rapid nuclear accumulation of the MR. Aldosterone-bound GFP-MR was concentrated in prominent clusters within the nucleus, whereas GFP-MR did not form clusters in the absence of hormone. Similar subnuclear distribution was observed with corticosterone, another MR agonist. In the presence of the MR antagonists spironolactone or ZK91587 the rate of nuclear translocation was significantly slower and the final nuclear-to-cytoplasmic ratio in steady state was significantly lower than with aldosterone. In addition, MR antagonists did not induce formation of nuclear GFP-MR clusters. MR antagonists also were able to disrupt pre-existing nuclear clusters formed in the presence of aldosterone. GFP-MR clusters were retained in nuclear matrix preparations after in vivo crosslinking. These data strongly suggest that hormone-activated MRs accumulate in dynamic discrete clusters in the cell nucleus, and this phenomenon occurs only with transcriptionally active mineralocorticoids.
The mineralocorticoid receptor (MR) is a member of the steroid-thyroid receptor superfamily. These receptors are ligand-dependent transcription factors generally located in the nucleus even in the absence of the hormone (see ref. 1 for review). The glucocorticoid receptor (GR), however, is an exception, because it is mainly cytoplasmic in the absence of ligand, and nuclear translocation occurs only after hormone binding (1). The subcellular localization of the MR is still unclear. Because the MR shows significant sequence homology to the GR, one would expect it to also be cytoplasmic in the absence of hormone. However, previous data on its localization are controversial. On the one hand, earlier biochemical and immunocytochemical studies found the unliganded MR to be cytoplasmic (2, 3). Similar findings were reported for the recombinant MR overexpressed in Sf9 insect cells and mouse macrophages (4). On the other hand, immunohistochemistry of kidney sections from adrenalectomized animals revealed both nuclear and cytoplasmic staining (2, 5, 6). In addition, a study with enucleated GH3 cells localized MR entirely to the nucleus (7). All previous studies were done by using fixed and permeabilized cells and antibodies, and methodological differences might have contributed to the controversial results. Furthermore, these studies did not permit examination of the in vivo kinetics of receptor trafficking.
To determine the subcellular distribution of the MR in living cells, in the present study we used a green fluorescent protein (GFP)-MR chimera. GFP is an autofluorescent protein that can be used to study subcellular localization and trafficking of various proteins, without cell permeabilization, fixation, and the use of antibodies (8). We expressed a GFP-MR in mammalian cells lacking endogenous MR, and monitored its subcellular movements after ligand binding by using fluorescence time-lapse microscopy.
Another unsettled question in mineralocorticoid physiology is the mechanisms by which MR antagonists prevent the effects of aldosterone. In theory, they could block nuclear translocation of the MR or interfere with a step in signal transduction after nuclear translocation. Regarding nuclear translocation, early biochemical studies found that the MR antagonist spironolactone failed to generate nuclear receptor complexes (9). Similarly, a recent immunohistochemical study using transfected MR found no nuclear translocation in the presence of antagonists (3). On the other hand, there is autoradiographic evidence for nuclear binding of spironolactone (10). These diverging results can be explained, at least in part, by the different methods used to determine nuclear binding (i.e., isolated nuclei, autoradiography, immunocytochemistry). Therefore, the second goal of this study was to monitor the subcellular trafficking of the GFP-MR on treatment with MR antagonists and examine the fine subnuclear distribution of the MR by laser-scanning confocal microscopy. We report here that although cytoplasmic to nuclear translocation of the MR occurs with both mineralocorticoid agonists and antagonists, the kinetics of the translocation and the subnuclear distribution of the MR are strikingly different when liganded to hormone agonists vs. antagonists.
MATERIALS AND METHODS
Plasmids.
The GFP-MR plasmid (pEGFP-rMR), containing sequences encoding fluorescence-enhanced jellyfish GFP (8) fused to full-length rat MR (11), was constructed by inserting the rat MR cDNA fragment downstream of the GFP sequence into a BglII site within the polylinker of the plasmid pEGFP-C1 (CLONTECH; see ref. 12). In the resulting fusion protein the C terminus of EGFP is coupled to the N terminus of full-length MR through a Ser-Gly-Leu-Arg-Ser sequence.
Cell Culture and Transfection.
Chinese hamster ovary (CHO)-AA8 cells were grown in α-MEM medium containing 10% FetalClone II CHO serum (HyClone), Madin Darby Canine Kidney (MDCK), and monkey kidney CV1 cells in DMEM/F12 medium with 10% fetal bovine serum (FBS). To avoid the influence of possible corticosterone and cortisol contamination originating from the serum, cells were grown in media containing FBS that was charcoal-stripped four times. According to our measurements, this procedure eliminates >99.0% of glucocorticoids present in serum. CHO, CV1, or MDCK cells were grown in monolayers on small glass coverslips. Transient transfections were carried out by using Lipofectamine with 20 ng of the GFP-MR plasmid per coverslip.
Determination of Transcriptional Activity.
CV1 cells were transfected with 50 ng of TAT3-LUC reporter (13) and 500 ng of pEGFP-rMR or MR expression vector (6RMR), as described (14). Twenty nanograms of Rous sarcoma virus-β-galactosidase plasmid was included as an internal standard and the BlueScript KS− vector plasmid was used as carrier to bring the total amount of DNA to 1 μg. Fresh medium containing 5% stripped serum was added to cells 18 hr before transfection, and the cells were transfected by using 1 ml Opti-MEM I reduced serum medium containing Lipofectamine-DNA complex and incubated at 37°C. Five hours later, 1 ml of fresh DMEM containing 10% stripped fetal bovine serum was added without removing the transfection mixture, and the cells were kept at 30°C. Sixteen hours later, medium containing 2 nM aldosterone was added to one of two identical transfections; 24 hr later, cells were harvested and extracts were prepared as previously described (13). The extracts were assayed for luciferase activity at 30°C and were normalized to protein content and β-galactosidase activity (expression driven by the Rous sarcoma virus promoter).
Preparation of Nuclear Matrices.
CV1 cells grown on coverslips were treated with 2 nM aldosterone for 30 min at 37°C, then cells were incubated with the in vivo crosslinker, dithiobis (succinimidyl) propionate (DSP) (1 mM) in Hanks’ solution, for 10 min at 37°C, and then with 1 mM DSP in 100 mM Pipes buffer containing 0.5% Triton X-100, 1 mM EGTA, 4% polyethylene glycol 8000, pH 6.9 for 10 min at 37°C. Cells were washed, incubated with 0.5% Nonidet P-40, treated with DNase, and extracted with high salt as described in ref. 15.
Fluorescence Microscopy.
Six to 18 hr after transfection with GFP-MR, the medium was replaced with one containing no serum, and the coverslips were placed into a heated chamber under the fluorescence microscope. The cells were kept at 37°C during microscopy. The chamber was perfused with culture medium (control period) followed by MR agonists or antagonists as specified below. Fluorescence images were captured on a PXL cooled charge-coupled device camera (Photometrics, Tucson, AZ) attached to a Olympus IMT2 microscope equipped with an epifluorescence attachment and standard fluorescein isothiocyanate, 4′-6-diamidino-2-phenylindole (DAPI), and Texas Red filter sets, using a 60× planapo objective (N.A. 1.4, Nikon). For fluorescence confocal microscopy a Bio-Rad MRC-1024 Laser Scanning Confocal System was used.
RESULTS
The GFP-MR Fusion Protein Is Transcriptionally Active.
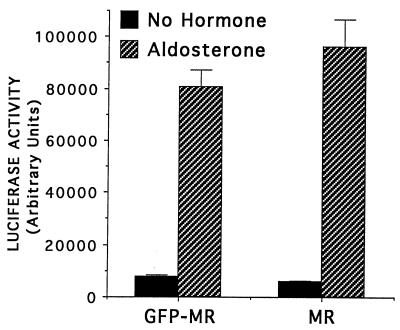
We initially wanted to determine whether the GFP-MR fusion protein behaved similarly to wild-type MR with respect to transcriptional activity. We therefore transfected CV1b cells (which lack endogenous MR; ref. 13) with the GFP-MR plasmid along with the MR-responsive reporter, TAT3-LUC. The transcriptional activity of GFP-MR at 30°C was consistently comparable to that of the wild-type MR (Fig. 1), whereas at 37°C, just like with the GR-GFP (16), it was <10% of that of the wild-type MR.
Figure 1.
GFP-MR transcriptional activity is comparable to wild-type MR. CV-1 cells were transiently transfected with the reporter plasmid TAT3-LUC containing three near-consensus hormone response elements upstream of a minimal promoter driving expression of the luciferase gene. Expression vectors for GFP-MR or wild-type MR were cotransfected. Cells were incubated for 16 hr with 2 nM aldosterone at 30°C, as indicated. Shown is the average of four separate transfections (±SEM) performed on the same day. Comparable results were obtained in three independent experiments performed on different days: the ratio of GFP-MR activity to wild-type MR activity varied by less than 15%.
Subcellular Distribution of GFP-MR in the Absence and Presence of Ligand.
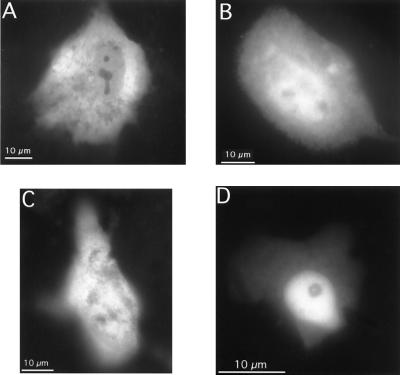
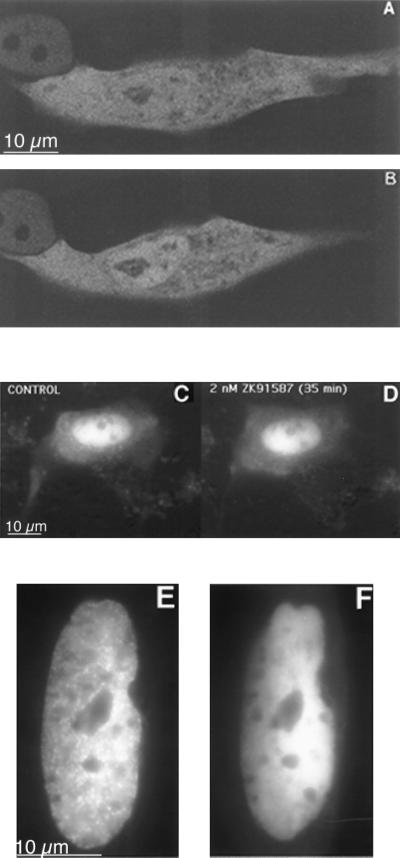
First we investigated the subcellular localization of GFP-MR in the absence of hormone, in living CHO, CV-1, and MDCK cells transiently transfected with GFP-MR. The majority of transfected cells exhibited both cytoplasmic and nuclear fluorescence (Fig. 2 A–C). However, in a small percent of cells GFP-MR seemed to be predominantly nuclear (Fig. 2D). No GFP-MR was observed in the nucleoli. This subcellular distribution pattern was similar when the GFP-MR construct was expressed in epithelial cells (MDCK; Fig. 2C) or nonepithelial cells (CHO or CV1; Fig. 2 A and B). Furthermore, the distribution pattern (cytoplasmic plus nuclear vs. predominantly nuclear) was unrelated to the level of GFP-MR expression (i.e., fluorescence intensity).
Figure 2.
Subcellular distribution of GFP-MR in the absence of hormone. CV1 cells (A and D), CHO cells (B), or MDCK cells (C) were transiently transfected with the GFP-MR plasmid and grown on coverslips in steroid-free medium at 37°C. Fluorescence images were captured on a PXL cooled charge-coupled device camera attached to a Olympus IMT2 microscope equipped with an epifluorescence attachment and standard fluorescein isothiocyanate filter set, using a 60× planapo objective (N.A. 1.4, Nikon).
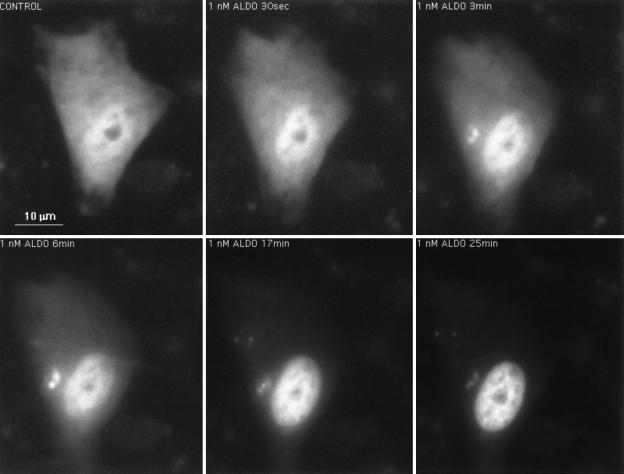
In the presence of aldosterone GFP-MR was completely localized to the nucleus in 100% of the transfected cells. The time course of nuclear translocation of GFP-MR after aldosterone binding was followed by real-time fluorescence cinematography. In cells where GFP-MR was mainly cytoplasmic in the absence of hormone, nuclear accumulation of GFP-MR started within 30 sec of addition of 1 nM aldosterone, was half-maximal at 7.5 min, and was complete in about 10 min (Fig. 3).§
Figure 3.
Time-dependent nuclear translocation of GFP-MR in the presence of aldosterone. CV1 cells transfected with the GFP-MR plasmid and grown on coverslips in steroid-free medium, were placed in a chamber maintained at 37°C with steroid-free medium (O min) or 1 nM aldosterone containing medium, and translocation of GFP-MR was followed in real time, as indicated. Nuclear accumulation of GFP-MR, started within 30 sec was half-maximal at 7.5 min and complete at 10 min.
Because corticosterone, an endogenous glucocorticoid, has the same affinity for the MR as aldosterone, we next tested if GFP-MR also translocates to the nucleus on binding of corticosterone, and if so, if it occurs with a kinetics similar to that of aldosterone. As shown in Fig. 4, incubation of transfected CV1 cells with 2 nM corticosterone also induced complete nuclear translocation of GFP-MR, and the rate of translocation was similar to that observed with aldosterone.
Figure 4.
Nuclear translocation of GFP-MR occurs with corticosterone as ligand. CV1 cells expressing GFP-MR and grown on coverslips were perfused with control medium (A) or 1 nM corticosterone for 20 min at 37°C (B).
Agonist-Liganded GFP-MR Is Localized to Clusters Within the Nucleus.
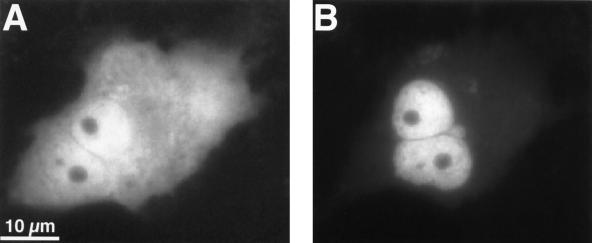
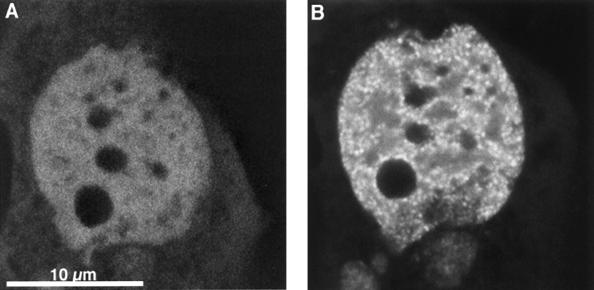
The subnuclear distribution pattern of aldosterone-activated GFP-MR was examined by confocal laser scanning microscopy. As shown in Fig. 5B, the distribution of aldosterone-bound GFP-MR within the nucleus is not homogenous, in that MRs are concentrated in prominent clusters. The number of these clusters, as determined by confocal microscopy (counting the number of local maxima within one 200-nm optical slice and then extrapolating for the total volume of the nucleus) was found to be between 1,500 and 4,500 per nucleus. Nucleoli were consistently negative. The punctate nuclear pattern appeared after about 5 min of aldosterone perfusion. Importantly, GFP-MR never formed clusters in the absence of hormone. In cells where GFP-MR was already nuclear without aldosterone, nuclear distribution was diffuse. In such cells, however, soon after aldosterone addition the GFP-MR molecules underwent a redistribution, resulting in the appearance of clusters (Fig. 5). When cells were incubated at 30°C with aldosterone, formation of nuclear clusters was very similar to that observed at 37°C (data not shown).
Figure 5.
GFP-MR forms nuclear clusters in the presence of aldosterone. Confocal images of a cell before (A) and 15 min after (B) perfusion with 1 nM aldosterone at 37°C. Formation of nuclear clusters started at about 5 min after aldosterone. Note that no nuclear clusters are present in the absence of aldosterone even when the MR is mainly nuclear before hormone addition.
Similar subnuclear distribution was observed when GFP-MR-expressing cells were perfused with corticosterone (Fig. 4B). Again, cluster formation started approximately 5 min after addition of corticosterone.
Subcellular and Subnuclear Distribution of GFP-MR on Binding of MR Antagonists.
The subcellular localization of GFP-MR differed markedly when cells were incubated with MR antagonists, both with respect to the rate and extent of translocation, and the subnuclear distribution pattern. The MR antagonists, spironolactone and ZK91587, did not cause a complete cytoplasmic-to-nuclear translocation of GFP-MR. Even with high concentrations of spironolactone (up to 1 μM) and after extended incubation periods (up to 60 min) about 45% of the GFP-MR remained in the cytoplasm (Fig. 6 A vs. B). The ratio of nuclear to cytoplasmic fluorescence was 28%, 31%, and 45% at 0, 10, and 35 min, respectively, after addition of 1 μM spironolactone. This process was even slower and less complete with ZK91587, although this latter compound has almost as high affinity for the MR as aldosterone (Kd ≈ 2 nM; ref. 17). In fact, in most cells there was very little ZK91587-induced nuclear translocation (Fig. 6 C and D). This observation is compatible with earlier results showing that the binding of ZK91587 does not induce activation of the MR (18).
Figure 6.
Subcellular localization of the GFP-MR in the presence of MR antagonists. Confocal images of two cells before (A) and after (B) incubation with 1 μM spironolactone for 45 min at 37°C. C and D are fluorescence microscopic images of a cell before (C) and after (D) incubation with 2 nM ZK91587 for 35 min. In all three cells nuclear translocation of GFP-MR was significantly less than that with aldosterone. (E) Cells were perfused with 1 nM aldosterone for 30 min; note prominent nuclear clusters. After 30 min, the perfusion medium was changed to 1 nM aldosterone plus 100 nM spironolactone, and the image on F was captured 30 min later. Note that spironolactone treatment disrupted the nuclear clusters.
Furthermore, in contrast to aldosterone, spironolactone or ZK91587 did not induce nuclear clustering of GFP-MR. Instead, GFP-MR accumulated in a diffuse manner throughout the nucleoplasm, but again nucleoli were negative. In those cells where the GFP-MR was nuclear in the absence of ligand, the nuclear distribution did not change on incubation with antagonists (Fig. 6 A and B), in strong contrast with the focal accumulation of GFP-MR observed in such cells in the presence of aldosterone (Fig. 5B). Thus, nuclear cluster formation of the MR could be observed only with transcriptionally active hormones and not with hormone antagonists.
Even more important, MR antagonists were able to disrupt clusters that formed in the presence of aldosterone. In these experiments live cells first were treated with 1 nM aldosterone, and then perfused with 1 μM spironolactone or ZK91587 in the continued presence of 1 nM aldosterone. As shown in Fig. 6F, the prominent focal accumulation of the GFP-MR observed with aldosterone (Fig. 6E) completely disappeared within 30 min after the addition of the MR antagonist.
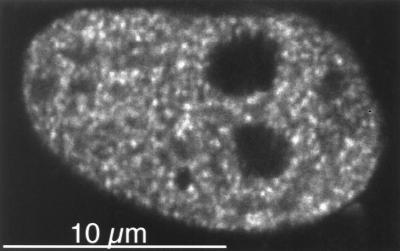
Nuclear GFP-MR Clusters Are Bound to the Nuclear Matrix.
Previous observations indicate that steroid receptors bind to the nuclear matrix when liganded with their respective hormones (15, 19, 20). Therefore we tested if GFP-MR clusters are present in nuclear matrix preparations from cells transiently transfected with GFP-MR and incubated with aldosterone. Because the interaction between GR and nuclear matrix seems relatively weak (15, 19) and can be detected only after crosslinking, we treated aldosterone-induced CV1 cells with the crosslinker dithiobis (succinimidyl) propionate before permealizing the cell membrane. Fig. 7 shows that the GFP-MR clusters were retained in these nuclear matrices. Although this observation indicates that MRs, like other steroid receptors, bind to the nuclear matrix in vivo, this interaction is probably weak, because it required crosslinking to be detected.
Figure 7.
Nuclear clusters are associated with the nuclear matrix. Confocal image of a CV1 cell incubated with 1 nM aldosterone for 30 min at 37°C, then treated with the in vivo crosslinker, dithiobis (succinimidyl) propionate (1 mM) for 10 min at 37°C, and then nuclear matrix extracted as described in Materials and Methods.
Costaining with DNA Dyes.
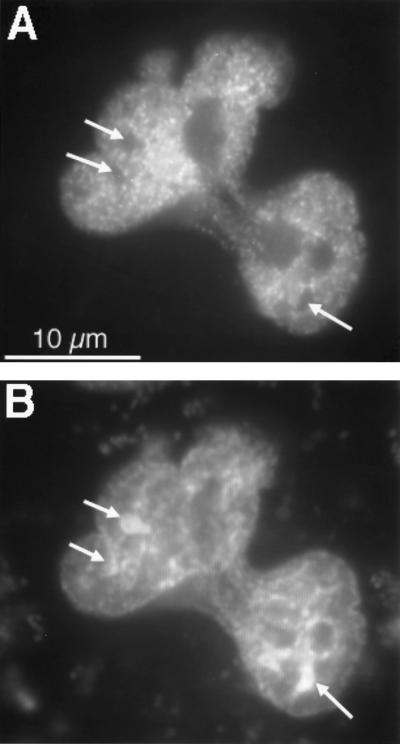
To address the question of whether the intranuclear distribution pattern of the GFP-MR correlates with the density of the chromatin, we costained aldosterone-treated cells with the DNA dyes DAPI or propidium iodide. As shown in Fig. 8, there was a negative correlation between the spatial distribution of GFP-MR and DAPI. Those regions that showed a strong DAPI staining, probably corresponding to the transcriptionally inactive heterochromatin, had few GFP-MR clusters. Similar negative correlation was observed when using propidium iodide instead of DAPI (data not shown). These observations suggest that the GFP-MR clusters preferentially accumulate on euchromatin and might be associated with the transcriptionally active regions of the chromatin.
Figure 8.
Comparison of the nuclear distribution of the DNA dye DAPI and GFP-MR. CV1 cells expressing GFP-MR were incubated with 1 nM aldosterone at 37°C for 30 min. Then the cells were fixed with 2% formaldehyde, permeabilized, and incubated with 2 μg/ml DAPI for 15 min at room temperature. (A) Intranuclear distribution of GFP-MR. (B) Intranuclear distribution of DAPI. Arrows point to the most dense DNA regions that are negative for GFP-MR.
DISCUSSION
In this study we used a GFP-MR fusion protein to examine the subcellular localization of MR, both in the absence of hormone as well as after treatment with agonists or antagonists. The use of GFP-tagged MR makes it possible to monitor intracellular trafficking in living cells, without the necessity of cell permeabilization and use of antibodies. Although a similar approach recently was applied to study the subcellular distribution of the GR (16, 21), subcellular trafficking of MR in living cells has not been examined by this approach.¶
Previous studies indicated that many proteins expressed as GFP fusions retain the same subcellular localization and biological activity as their native counterparts (8, 22, 23). Nevertheless, we thought it was important to establish that the GFP-MR fusion protein remains functional. Our experiments with transfected cells demonstrate that the GFP-MR fusion protein is able to specifically bind aldosterone, and on TAT3-LUC reporter gene it is as active as the wild-type MR, indicating that the presence of the GFP sequence at the N terminus of MR did not alter its biological activity significantly. This conclusion also is supported by the observation that the GFP-MR protein exhibited concentration-dependent nuclear translocation in the presence of the physiological hormone, aldosterone, and practically all GFP-MR molecules were able to translocate.
Studies on the subcellular localization of the MR in the absence of steroids have been controversial. There are data suggesting either exclusively cytoplasmic (3, 4) or nuclear (7) localization. Studying living cells, we found that in the absence of ligand GFP-MR was localized to both the cytoplasm and the nucleus in most cells. This observation would put the MR in a third category within the steroid/thyroid receptor superfamily, in which the unliganded receptor is not exclusively cytoplasmic like the GR (21) or nuclear as other members of this family (1), but rather it localizes to both compartments. A constant shuttling of unliganded receptors between the cytoplasm and nucleus has been suggested for other steroid receptors (20, 24), and such a mechanism is likely to contribute to the observed localization of the unliganded GFP-MR in this study. However, we also have observed a few cells where the GFP-MR was nuclear even in the absence of hormone. Because the fluorescence intensity was similar in cells in which the unliganded GFP-MR was predominantly cytoplasmic vs. nuclear, different levels of expression are unlikely to explain this heterogeneity. One possible explanation is that the subcellular distribution of the MR changes during the cell cycle, and because our cells were not synchronized, this could have resulted in the observed heterogeneity. Alternatively, cell-to-cell variation in the expression levels of other proteins necessary for MR nuclear localization might be responsible for the heterogenous distribution. Although we used serum that was charcoal-stripped four times, and therefore >99% of endogenous steroids were removed, the possibility that nonsteroidal activation of the MR occurred because of some components of the medium cannot be excluded.
Perhaps the most important finding of the present study is that the subnuclear distribution of MRs is heterogenous in the presence of agonists. Within the nucleus the receptors accumulate in clusters, and such clusters form only with hormones that can activate transcription. We found that both aldosterone and corticosterone, which are MR agonists, induced nuclear clusters, whereas the MR antagonists spironolactone and ZK91587 did not. The subnuclear distribution pattern of the agonist-liganded GFP-MR is remarkably similar to the pattern of the GR in the presence of dexamethasone observed either by immunohistochemistry on fixed cells (15, 25) or using a GFP-GR fusion protein in live cells (21). Furthermore, Htun et al. (21) found no nuclear clusters in the presence of the GR-antagonist RU486. van Steensel et al. (26) also observed nuclear clusters of the MR in fixed hippocampal tissue by using immunohistochemistry, but distribution of the MR was not affected by adrenalectomy or hormone replacement. We suspect that the lack of effect of hormonal conditions on MR distribution in that study (26) might have been caused by the different specificity of the antibody for activated vs. nonactivated receptor.
The exact nature and the function of the nuclear clusters is presently unknown. Htun and coworkers (21) suggested that with the GR such clusters correspond to activated target genes. However, optically detectable cluster formation requires probably many more MR molecules than the number of MRs expected to be associated with the promoter region of aldosterone-induced genes. Furthermore, it seems unlikely that aldosterone would increase the transcription of 1,500–4,500 genes (the number of GFP-MR clusters). Therefore, we feel that these clusters cannot represent only target genes. In agreement with this idea, van Steensel et al. (15) reported that in the hippocampus there was no correlation between the distribution pattern of GR clusters and the distribution of newly synthesized pre-mRNA, again arguing that the GR clusters are not directly associated with transcriptional units.
Why then do we see a correlation between transcriptional activity and the ability to induce cluster formation of a given steroid? We propose that the observed focal distribution of the aldosterone-liganded MR represents a necessary step leading to transcriptional activation. For example, nuclear clusters (which, as our data show, are still present in nuclear matrix preparations), may bind to chromatin regions that act as “ushers” to direct the activated receptors to the appropriate genes. The clustered binding of the MR to such nuclear acceptor sites might be important in scanning for aldosterone target genes. Recently Tang and DeFranco (20) proposed a dynamic model for the GR, speculating that a rapid association/dissociation of the GR with the nuclear matrix would facilitate an effective scanning for steroid target genes.
Our data also revealed that MR antagonists can interfere with aldosterone action by at least two mechanisms: the nuclear translocation rate of antagonist-liganded GFP-MR is significantly slower than that observed with aldosterone, and they are not able to induce nuclear cluster formation of the MR. Our results also indicate that nuclear cluster formation is rapidly reversible. When spironolactone or ZK91587 was added after the typical focal accumulation of MR-aldosterone complexes, the clusters disappeared within 30 min, and nuclear distribution of GFP-MR became homogenous. These data suggest that nuclear cluster formation is a critical step leading to transcriptional activity. This conclusion also is supported by our observation that the GFP-MR clusters preferentially accumulate over regions that show less intense staining with DNA dyes, probably corresponding to the transcriptionally more active euchromatin. Previous electron microscopic studies found that the progesterone receptor was associated with transcriptionally inactive condensed chromatin in the absence of progesterone, whereas it was distributed over less condensed chromatin after hormone addition (1).
In summary, our data strongly suggest that hormone-activated MRs accumulate in discrete clusters in the cell nucleus, and this phenomenon occurs only with transcriptionally active mineralocorticoids. The nature of nuclear clusters, their acceptor sites, and role in the mechanism of aldosterone action remain to be determined.
Acknowledgments
We thank Emily S. Cleaveland (Dartmouth Medical School) and Jian Wang (University of California, San Francisco) for technical assistance. This work was supported by National Institutes of Health Grants DK39523, DK41841, and DK51151. Confocal imaging was done at Dartmouth Medical School in The Herbert C. Englert Cell Analysis Laboratory, which was established by a grant from the Fannie E. Rippel Foundation and is supported in part by the Core Grant of the Norris Cotton Cancer Center (CA 23108).
ABBREVIATIONS
- MR
mineralocorticoid receptor
- GFP
green fluorescent protein
- GR
glucocorticoid receptor
- CHO
Chinese hamster ovary
- DAPI
4′,6-diamidino-2-phenylindole
- MDCK
Madin-Darby Canine Kidney
Footnotes
While this study was in progress, nuclear localization of GFP-MR was described in transfected A6 cells, however, subnuclear distribution was not investigated (12).
A quicktime movie of GFP-MR nuclear localization can be seen at the Mineralocorticoid Receptor Resource (MRR). The URL is: http://gladstone.ucsf.edu/givi/pearce/mrr.html.
References
- 1.Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E. J Steroid Biochem Mol Biol. 1996;56:3–9. doi: 10.1016/0960-0760(95)00268-5. [DOI] [PubMed] [Google Scholar]
- 2.Lombes M, Farman N, Oblin M E, Baullieu E E, Bonvalet J P, Erlanger B F, Gasc J M. Proc Natl Acad Sci USA. 1990;87:1086–1088. doi: 10.1073/pnas.87.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lombes M, Binart N, Baullieu E E, Rafestin-Oblin M E. Biochem J. 1994;302:191–197. doi: 10.1042/bj3020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson N, Schulman G, Karnik S, Alnemri E, Litwack G. Mol Endocrinol. 1993;7:1226–1239. doi: 10.1210/mend.7.9.8247024. [DOI] [PubMed] [Google Scholar]
- 5.Farman N, Oblin M E, Lombes M, Delahaye F, Westphal H M, Bonvalet J P, Gasc J M. Am J Physiol. 1991;260:C226–C233. doi: 10.1152/ajpcell.1991.260.2.C226. [DOI] [PubMed] [Google Scholar]
- 6.Sasano H, Fukushima K, Sasaki I, Matsuno S, Nagura H, Krozowski Z S. J Endocrinol. 1992;132:305–310. doi: 10.1677/joe.0.1320305. [DOI] [PubMed] [Google Scholar]
- 7.Pearce P T, McNally M, Funder J W. Clin Exp Pharmacol Physiol. 1986;13:647–654. doi: 10.1111/j.1440-1681.1986.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 8.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 9.Marver D, Stewart J, Funder J W, Feldman D, Edelman I S. Proc Natl Acad Sci USA. 1974;71:1431–1435. doi: 10.1073/pnas.71.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonvalet J P, Blot-Chabaud M, Farman N. Endocrinology. 1991;128:280–284. doi: 10.1210/endo-128-1-280. [DOI] [PubMed] [Google Scholar]
- 11.Patel P D, Sherman T G, Goldman D J, Watson S J. Mol Endocrinol. 1989;3:1877–1885. doi: 10.1210/mend-3-11-1877. [DOI] [PubMed] [Google Scholar]
- 12.Chen S-Y, Wang J, Liu W-H, Pearce D. Am J Physiol. 1998;274:C39–C46. doi: 10.1152/ajpcell.1998.274.1.C39. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Wang J, Sauter N K, Pearce D. Proc Natl Acad Sci USA. 1995;92:12480–12484. doi: 10.1073/pnas.92.26.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Wang J, Yu G, Pearce D. Mol Endocrinol. 1996;10:1399–1406. doi: 10.1210/mend.10.11.8923466. [DOI] [PubMed] [Google Scholar]
- 15.van Steensel B, Brink M, van der Meulen K, van Binnendijk E P, Wansink D G, de Jong L, de Kloet E R, van Driel R. J Cell Sci. 1995;108:3003–3011. doi: 10.1242/jcs.108.9.3003. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa H, Inouye S, Tsuji F I, Yasuda K, Umesono K. Proc Natl Acad Sci USA. 1995;92:11899–11903. doi: 10.1073/pnas.92.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutanto W, DeKloet E R. Life Sci. 1988;43:1537–1543. doi: 10.1016/0024-3205(88)90402-x. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal M K, Kalimi M. Biochem Biophys Res Comm. 1988;150:449–455. doi: 10.1016/0006-291x(88)90541-4. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann S H, Okret S, Wilstrom A C, Gustafsson J-A, Shaper J H. J Biol Chem. 1986;261:11962–11967. [PubMed] [Google Scholar]
- 20.Tang Y, DeFranco D B. Mol Cell Biol. 1996;16:1989–2001. doi: 10.1128/mcb.16.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Htun H, Barsony J, Renyi I, Gould D L, Hager G L. Proc Natl Acad Sci USA. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Hazelrigg T. Nature (London) 1994;369:400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- 23.Inouye S, Tsuji F I. FEBS Lett. 1994;341:277–280. doi: 10.1016/0014-5793(94)80472-9. [DOI] [PubMed] [Google Scholar]
- 24.Guiochon-Mantel A, Milgrom E. Trends Endocrinol Metab. 1993;4:322–328. doi: 10.1016/1043-2760(93)90074-o. [DOI] [PubMed] [Google Scholar]
- 25.Martins V R, Pratt W B, Terracio L, Hirst M A, Ringold G M, Housley P R. Mol Endocrinol. 1991;5:217–225. doi: 10.1210/mend-5-2-217. [DOI] [PubMed] [Google Scholar]
- 26.van Steensel B, van Binnendijk E P, Hornsby C D, van der Voort H T, Krozowski Z S, de Kloet E R, van Driel R. J Cell Sci. 1996;109:787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]