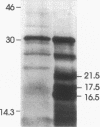
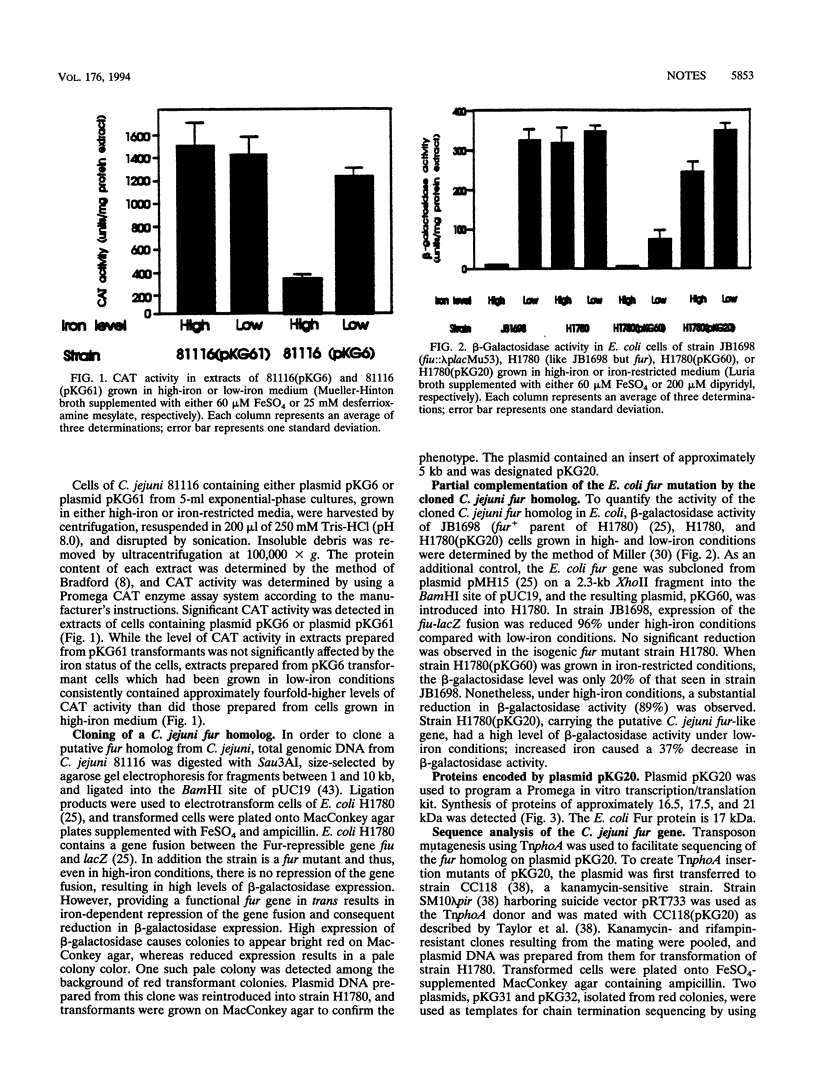
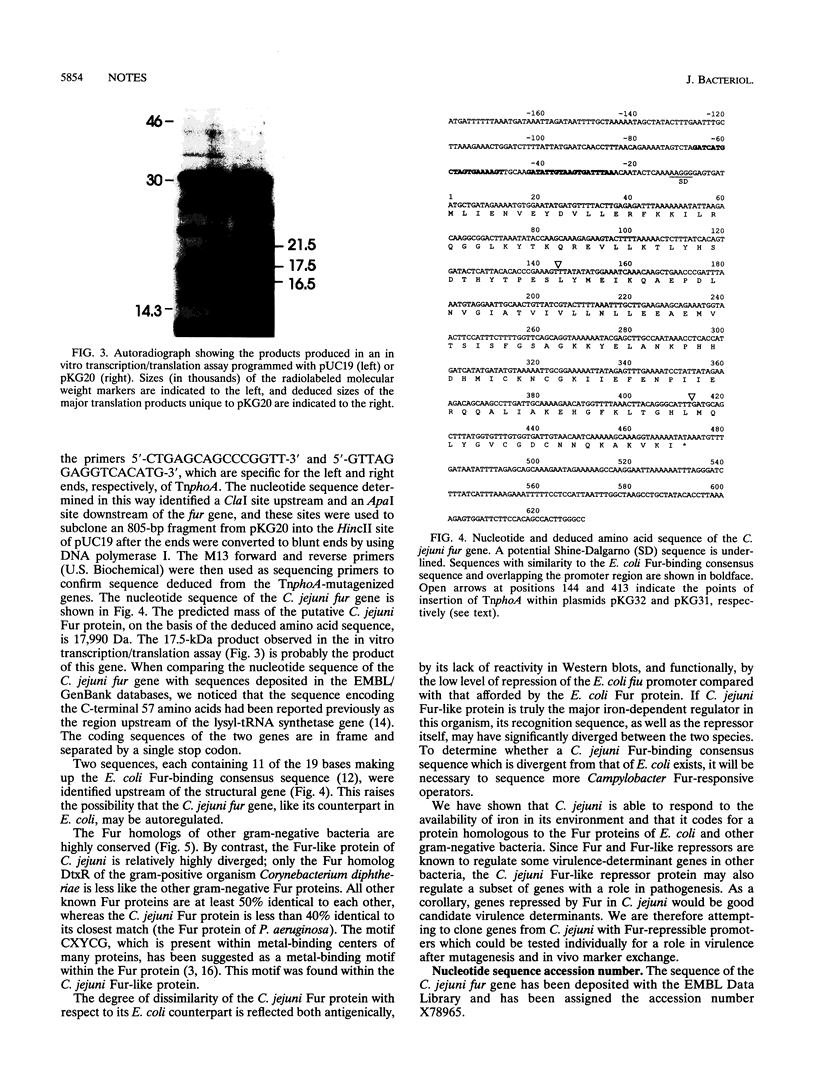
Abstract
The Fur protein of Escherichia coli represses transcription from Fur-responsive genes in an iron-dependent manner. We have demonstrated a Fur-like iron-responsive genetic regulatory activity operating in Campylobacter jejuni by using a chloramphenicol acetyl transferase reporter gene separated from its promoter by a synthetic Fur-responsive operator. A fur-like gene has been cloned from C. jejuni by partial functional complementation of an E. coli fur mutation. Sequence analysis has shown that, at the amino acid level, the C. jejuni Fur protein is 35% identical with its E. coli counterpart.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm R. A., Guerry P., Trust T. J. The Campylobacter sigma 54 flaB flagellin promoter is subject to environmental regulation. J Bacteriol. 1993 Jul;175(14):4448–4455. doi: 10.1128/jb.175.14.4448-4455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berish S. A., Subbarao S., Chen C. Y., Trees D. L., Morse S. A. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect Immun. 1993 Nov;61(11):4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988 Feb;170(2):1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M. B., Guerry P., Lee E. C., Burans J. P., Walker R. I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985 Dec;50(3):941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. L., Bingham H. L. Lysyl-tRNA synthetase gene of Campylobacter jejuni. J Bacteriol. 1992 Feb;174(3):695–701. doi: 10.1128/jb.174.3.695-701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., James L. P., Helmann J. D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993 Sep;175(17):5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy M., Neilands J. B. Structural dynamics and functional domains of the fur protein. Biochemistry. 1991 Aug 20;30(33):8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Darling W. M., Peel R. N., Skirrow M. B., Mulira A. E. Campylobacter cholecystitis. Lancet. 1979 Jun 16;1(8129):1302–1302. doi: 10.1016/s0140-6736(79)92269-4. [DOI] [PubMed] [Google Scholar]
- Davies J. S., Penfold J. B. Campylobacter urinary infection. Lancet. 1979 May 19;1(8125):1091–1092. doi: 10.1016/s0140-6736(79)92995-7. [DOI] [PubMed] [Google Scholar]
- Field L. H., Headley V. L., Payne S. M., Berry L. J. Influence of iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect Immun. 1986 Oct;54(1):126–132. doi: 10.1128/iai.54.1.126-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., Henocque G., Kremp L., Rocque J., Boury R., Alanio G., Vlaes L., Hemelhof W., Van den Borre C., Macart M. Nosocomial outbreak of Campylobacter jejuni meningitis in newborn infants. Lancet. 1986 Jul 19;2(8499):146–149. doi: 10.1016/s0140-6736(86)91956-2. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Harris L. A., Logan S. M., Guerry P., Trust T. J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987 Nov;169(11):5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin C. M., Boyko S. A., Calderwood S. B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992 Mar;174(6):1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin C. M., Calderwood S. B. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993 Feb;175(3):706–715. doi: 10.1128/jb.175.3.706-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascart G., Gottignies P. Enteritis and septicaemia due to Campylobacter jejuni. Acta Clin Belg. 1979;34(6):365–369. doi: 10.1080/22953337.1979.11718711. [DOI] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Dolby J. M. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J Hyg (Lond) 1985 Oct;95(2):217–227. doi: 10.1017/s0022172400062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Auffenberg T., Pesci E. C., Sheen V. L., Jusuf S. S. Iron acquisition and hemolysin production by Campylobacter jejuni. Infect Immun. 1992 Sep;60(9):3872–3877. doi: 10.1128/iai.60.9.3872-3877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. W., Cox C. D., Vasil M. L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993 May;175(9):2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991 Jan;173(2):417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Holmes R. K. Iron regulation of the cloned diphtheria toxin promoter in Escherichia coli. Infect Immun. 1988 Sep;56(9):2430–2436. doi: 10.1128/iai.56.9.2430-2436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. A., Wang L. L., West A., Sparling P. F. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J Bacteriol. 1993 Feb;175(3):811–818. doi: 10.1128/jb.175.3.811-818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbeser L. S., Tolmasky M. E., Actis L. A., Crosa J. H. Mechanisms for negative regulation by iron of the fatA outer membrane protein gene expression in Vibrio anguillarum 775. J Biol Chem. 1993 May 15;268(14):10433–10439. [PubMed] [Google Scholar]
- Wang Y., Taylor D. E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990 Sep 28;94(1):23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- Wassenaar T. M., Bleumink-Pluym N. M., van der Zeijst B. A. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991 Aug;10(8):2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]