Abstract
We have derived a cardiac muscle cell line, designated HL-1, from the AT-1 mouse atrial cardiomyocyte tumor lineage. HL-1 cells can be serially passaged, yet they maintain the ability to contract and retain differentiated cardiac morphological, biochemical, and electrophysiological properties. Ultrastructural characteristics typical of embryonic atrial cardiac muscle cells were found consistently in the cultured HL-1 cells. Reverse transcriptase–PCR-based analyses confirmed a pattern of gene expression similar to that of adult atrial myocytes, including expression of α-cardiac myosin heavy chain, α-cardiac actin, and connexin43. They also express the gene for atrial natriuretic factor. Immunohistochemical staining of the HL-1 cells indicated that the distribution of the cardiac-specific markers desmin, sarcomeric myosin, and atrial natriuretic factor was similar to that of cultured atrial cardiomyocytes. A delayed rectifier potassium current (IKr) was the most prominent outward current in HL-1 cells. The activating currents displayed inward rectification and deactivating current tails were voltage-dependent, saturated at ≫+20 mV, and were highly sensitive to dofetilide (IC50 of 46.9 nM). Specific binding of [3H]dofetilide was saturable and fit a one-site binding isotherm with a Kd of 140 +/− 60 nM and a Bmax of 118 fmol per 105 cells. HL-1 cells represent a cardiac myocyte cell line that can be repeatedly passaged and yet maintain a cardiac-specific phenotype.
Keywords: simian virus 40 T oncogene, potassium channels, dofetilide
Attempts to create a cardiac muscle cell line that proliferates in culture while maintaining a differentiated phenotype have to date not been successful. One such effort gave rise to AT-1 cells, which were derived from an atrial tumor growing in a transgenic mouse in which expression of the simian virus 40 (SV40) large T antigen was targeted to atrial cardiomyocytes via the atrial natriuretic factor (ANF) promoter (1–5). These highly differentiated myocytes have been maintained for over 6 years by serial propagation as ectopic grafts in syngeneic mice. AT-1 cells can be cultured and they maintain their cardiac phenotype, but they cannot be passaged in culture or recovered from frozen stocks and thus must be used as primary cells.
Other attempts to immortalize cardiac myocytes with SV40 large T antigen have used a variety of cardiac promoters in transgenic animals (5–7) or have used retroviral transfection of primary cells (8–10). Still other approaches have made use of the protooncogenes v-Myc and v-H-Ras to transform cardiac myoblasts (11, 12). Differentiation of precardiac splanchnic mesoderm also has been attempted (13). In each of these cases, continued passage in culture has resulted in the gradual loss of either the cardiac phenotype or the ability to proliferate.
In this report we describe the establishment of a cardiac myocyte cell line that: (i) can be passaged indefinitely in culture, (ii) can be recovered from frozen stocks, (iii) retains a differentiated cardiac myocyte phenotype, and (iv) maintains contractile activity. We further identify in these cells electrophysiological and pharmacological features that are characteristic of cardiac myocytes and that demonstrate the utility of these cells as an experimental system for the study of cardiac function.
MATERIALS AND METHODS
Isolation and Culture of HL-1 Cells.
The HL-1 cell line was established from an AT-1 subcutaneous tumor excised from an adult female Jackson Laboratory-inbred C57BL/6J mouse. The tumor tissue was removed from the euthanized animal and carefully trimmed of connective tissues. The excised tissue was minced and incubated at 4°C for 14 hr in 0.125% trypsin in Joklik’s medium with gentle agitation (4). Cells were obtained by sequential digestions with 0.1% collagenase in Joklik’s medium at 37°C, pooled, and plated (15 × 106 cells/5 ml) in gelatin/fibronectin-coated T25 flasks (fibronectin from Sigma, gelatin from Difco). To coat the flasks, 25 μg of fibronectin in 2 ml of 0.02% gelatin in water was added to each T25 flask, which was incubated at 37°C overnight. The cells were maintained in Ex-Cell 320 medium (JRH Biosciences, Lenexa, KS) supplemented with 10% fetal bovine serum (BioWhittaker), 10 μg/ml insulin (Life Technologies, Grand Island, NY), 50 μg/ml endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, NY), 1 μM retinoic acid (Sigma), 10 μM norepinephrine (Sigma), 100 units/ml penicillin, 100 μg/ml streptomycin (Life Technologies), and an additional 1× nonessential amino acids (Life Technologies). The medium was changed approximately every 24 hr. The cells were grown at 37°C in an atmosphere of 5% CO2 and 95% air at a relative humidity of approximately 95%. Once the HL-1 cells reached confluence, the cell cultures were split 1 to 3, and this was designated as a passage. The HL-1 cell line tested negative for mycoplasma contamination at passages 13, 51, and 96 (Bionique Testing Laboratory, Saranac Lake, NY).
Immunohistochemistry and Confocal Microscopy.
For indirect immunofluorescence HL-1 cells grown on gelatin/fibronectin-coated coverslips were fixed with 2.5% paraformaldehyde in PBS (pH 7.2) for 15 min at 4°C and permeabilized with 0.1% Triton X-100 for 3 min at room temperature. The primary antibodies used for indirect immunofluorescent staining were: monoclonal anti-desmin from Sigma (catalogue no. D1033), and mAbs MF 20 to sarcomeric myosin heavy chain (MHC) (kindly provided by D. M. Bader, Vanderbilt University) and to ANF (kindly provided by C. C. Glembotski, University of California, San Diego). The secondary antibody was either rhodamine- or fluorescein-conjugated goat anti-mouse IgG (Sigma). Cultures were examined on a Leica confocal laser scanning microscope equipped with a Polaroid digital image capture system and recorded on Kodak Gold 100 film. Images are printed in false color.
Transmission Electron Microscopy.
Selected cultures were prepared for electron microscopy in situ as described previously (14). Cells were fixed in 4.0% glutaraldehyde/0.1 M sodium cacodylate, postfixed in 1.0% osmium tetroxide/0.1 M sodium cacodylate, stained enbloc by using 0.5% aqueous uranyl acetate. This process was followed by dehydration in a graded alcohol series, with infiltration and embedment using Polybed 812 plastics (Polysciences). Thin sections were obtained by using a Reichert Ultracut Ultramicrotome equipped with a diamond knife, collected on uncoated 200-mesh copper grids, poststained with lead citrate, and examined in a JEOL 1210 transmission electron microscope at 60 kV.
Detection of Contractile Protein Isoforms, ANF, and Connexin43 Gene Expression.
Reverse transcription–PCR (RT-PCR)-based assays were used to determine the pattern of gene expression in passage 86 cultures of HL-1 cells. For each assay, total RNA was isolated by using TRIzol (Life Technologies). RT-PCR was done in a 60-μl reaction mixture containing 50 mM KCl, 10 mM Tris⋅HCl (pH 8.4), 2.5 mM MgCl2, 20 μl/ml 1% gelatin, 200 μM each dNTP, 100 pmols of each oligonucleotide primer, 2 units of Taq DNA polymerase, 40 units RNasin, 2 units of avian myeloblastosis virus reverse transcriptase (Life Technologies), and 1.5 μg of total RNA. The amplification programs, primers, and controls were the same as previously described (4) except that a Perkin–Elmer GeneAmp 9600 PCR system was used and the incubations at each temperature were carried out for only 30 sec.
Electrophysiological Recording.
HL-1 cells from passages 60 to 80 were grown on 5-mm glass coverslips (Bellco Glass) coated with gelatin/fibronectin. Current recordings were performed with an Axopatch one-dimensional patch-clamp amplifier (Axon Instruments, Foster City, CA) in the whole-cell configuration of the patch-clamp technique (15). Data acquisition and command potentials were controlled with a commercial software program (pclamp, Axon Instruments). The external solution was normal Tyrode’s solution and contained: 130 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH adjusted to 7.35 with NaOH). The internal (pipette) solution contained: 110 mM KCl, 5 mM K2ATP, 5 mM K4BAPTA, 1 mM MgCl2, and 10 mM Hepes (pH adjusted to 7.2 with KOH). In experiments designed to record outward potassium currents, L-type calcium current was eliminated by the addition of cadmium (200 μM) to the extracellular solution. Sodium and T-type calcium currents were inactivated by the holding potential of −50 mV. Microelectrodes were pulled from borosilicate glass (Garner Glass, Claremont, CA) and heat-polished (pipette tip resistance, 5–9 MΩ). Ion currents were recorded at room temperature (22–23°C).
[3H]Dofetilide Binding.
[3H]dofetilide (38 Ci/mmol) was synthesized by catalytic tritiation (New England Nuclear) of N-[3-bromo-4-[2-[methyl[2-[3-bromo-4-[(methylsulfonyl)-amino]phenyl]ethyl]amino]ethoxy]phenyl]-methanesulfonamide, obtained by bromination of dofetilide hydrochloride with Br2 in glacial acetic acid at 0°C. HL-1 cells from passages 60 to 80 were dissociated from tissue culture flasks with 0.1% trypsin (Clonetics, San Diego), and [3H]dofetilide binding experiments were performed by using a modification of the method by Chadwick et al. (16). The cells were suspended in a buffer containing: 40 mM KCl, 20 mM KH2PO4, 5 mM MgCl2, 0.5 mM KHCO3, 10 mM glucose, 50 mM potassium glutamate, 20 mM potassium aspartate, 1 mM EGTA, and 10 mM Hepes, adjusted to pH 7.4 with KOH and containing 0.1% BSA and incubated with [3H]dofetilide for 45 min at 37°C. The cells then were filtered on GF/C Unifilter 96-well filter plates (Packard) by using a Packard Micromate 496 harvester. Filter plates were presoaked with wash buffer containing: 25 mM Tris⋅HCl (pH 7.4), 130 mM NaCl, 5.5 mM KCl, 0.8 mM MgCl2, 0.05 mM CaCl2, and 1.0% (wt/vol) BSA and washed after harvesting at room temperature with 4 × 1-ml washes of the same buffer without albumin. Bound [3H]dofetilide was determined after addition of Microscint-20 (Packard) by liquid scintillation spectroscopy in a Packard TopCount Scintillation Counter. Specific binding was analyzed by nonlinear regression fit using GraphPad Prism software (GraphPad, San Diego).
RESULTS
Establishment of the HL-1 Cell Line.
The HL-1 cell line was isolated from a culture of AT-1 cells after more than 100 separate preparations and attempts to passage AT-1 cells in vitro and by very carefully controlling the culture environment. The HL-1 cell line can be serially propagated in culture with an apparently unlimited life span. Moreover, HL-1 cells, under selective culture conditions, retain the ability to contract through at least passage 240. These characteristics are maintained in cells that have been recovered from stocks frozen in liquid nitrogen. We have determined after exhaustive studies that to maintain these cells in a highly proliferative and differentiated beating state that they must be cultured under the conditions summarized in Materials and Methods. These conditions include using Ex-Cell 320 medium as the basic culture medium. Other media that we tested and tried to formulate could not maintain these cellular characteristics nearly as well as the Ex-Cell 320 medium. Also, endothelial cell growth supplement, which is a mixture of growth and attachment factors, is added to support cell division. Retinoic acid and norepinephrine are necessary to maintain a differentiated phenotype and to stimulate beating. Many other growth factors and agents were tested, and this formulation was determined to be the minimal growth medium for maintaining the capacity of the HL-1 myocytes to be passaged and to remain differentiated and beating.
We have determined that HL-1 cell cultures at passages beyond 30 to be a homogeneous cell population. The cultures contain no other cell type. This determination was established by triple-staining with Hoechst 33258 to stain nuclei and anti-desmin and anti-sarcomeric myosin antibodies to determine whether the stained nuclei were contained in a muscle cytoplasm. Every cell that contains a nucleus also stains for desmin and sarcomeric myosin (data not shown).
Morphological Characterization.
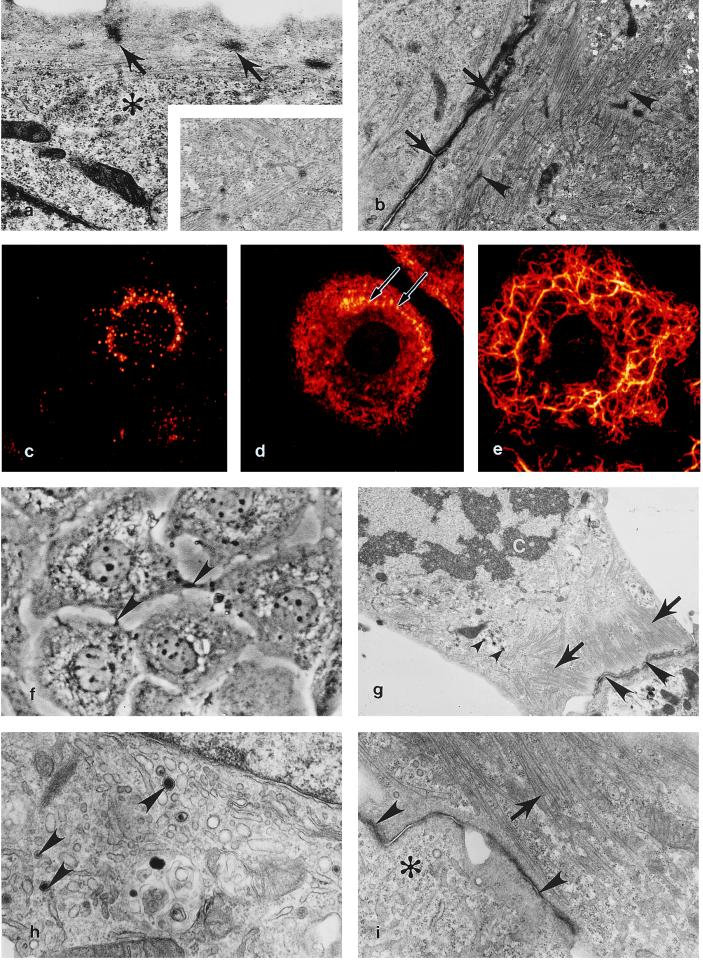
The ultrastructure of HL-1 cells resembles AT-1 cells and immature mitotic mouse atrial cardiomyocytes in situ. As is typical of healthy mitotically active cardiac muscle cells, the cytoplasm of HL-1 cells is filled with nascent myofibrils (Fig. 1 a and b) and areas rich in glycogen (Fig. 1a). Most HL-1 cells contain a single centrally positioned nucleus surrounded by a circular array of contracting myofibrils (Fig. 1f). At all passages studied (10, 20, 34, and 86), HL-1 cells possessed perinuclear ANF-containing specific granules (Fig. 1 c, g, and h), cardiac-specific myosin (Fig. 1d), and muscle-specific desmin intermediate filaments (Fig. 1e). Myofibrillogenesis was observed in HL-1 cells undergoing reorganization after either cell passage or cytokinesis. Nascent myofibrils first appeared as subplasmalemmal punctate Z densities linked by several thick and thin filaments (Fig. 1 a and d). This finding is typical of normal myofibril development in mitotic cardiomyocytes in the developing heart (17). During mitosis, HL-1 cells remained attached to adjacent cells via intercalated discs (Fig. 1 f, g, and i) and contained peripheral myofibrils consisting of partial sarcomeres lacking definitive Z lines (Fig. 1g). These characteristics also are seen in cultured cardiomyocytes and in mitotically active atrial cardiomyocytes in situ.
Figure 1.
Expression of a cardiac-specific phenotype at multiple passages in the HL-1 cardiomyocyte cell line. (a) A 10th-passage HL-1 cell demonstrating subsarcolemmal Z densities (arrows) typical of normal myofibrillogenesis in cultured and in vivo cardiomyocytes, glycogen (∗). (×12,500.) (Inset) High-magnification view demonstrating typical cardiac-specific myofibril banding. (×20,000.) (b) Two 10th-passage HL-1 cells containing myofibrils at various stages of sarcomerogenesis (arrowheads) are attached via an immature intercalated disc (arrows). (×9,670.) (c) Immunofluorescent localization of ANF expression in a passage 20 HL-1 cell. (d) Myosin is localized to scattered filaments in the cytoplasm and to thin reorganizing myofibrils located along the peripheral cytoplasm (arrows) in a passage 20 HL-1 cell. (e) In a passage 20 HL-1 cell desmin is expressed as reticulated cytoplasmic rings of intermediate filaments extending into lamellapodia. (c–e) False color, indirect immunofluorescence confocal laser scanning microscopy images. (×1,650.) (f) Passage 34 HL-1 cells demonstrating centrally located mononucleation and intercellular junctional contacts (arrowheads); phase-contrast, ×700. (g) At passage 34, dividing HL-1 cells behave like typical mitotic cardiomyocytes in that they retain peripheral myofibrils (arrows), contain atrial granules (small arrowheads,) and are anchored to adjacent cardiomyocytes by intercalated discs (arrowheads). C, chromosomes. (×8,000.) (h) At passage 86, an active Golgi and atrial-specific granules are present (arrowheads). (×25,000.) (i) A passage 86 HL-1 cell containing organized myofibrils (arrow), an intercalated disc (arrowheads), and areas occupied by free ribosomes (∗). (×15,000.) (a, b, and g–i) Transmission electron micrographs.
Gene Expression in the HL-1 Cell Line.
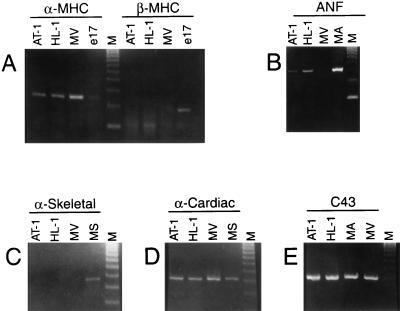
To characterize the HL-1 cell line in terms of the cardiac-specific genes that it expresses and as a further assessment of its differentiated phenotype, Reverse transcription–PCR (RT-PCR)-based analyses were used. Contractile protein isoform expression is a well-established index of the developmental and differentiated state of cardiomyocytes. As shown in Fig. 2, HL-1 cells expressed the adult isoform of MHC (α-MHC) whereas the control (embryonic ventricle) expressed only the embryonic isoform of myosin (β-MHC). Cultured HL-1 cells also expressed α-cardiac actin similar to adult mouse ventricle cells. In addition, HL-1 cells expressed transcripts for ANF and connexin43 (the major protein of the adult gap junction). This RT-PCR analysis demonstrated that the pattern of gene expression of cultured HL-1 cells was similar qualitatively to that of adult atrial cardiomyocytes.
Figure 2.
Reverse transcriptase–PCR-based analyses of gene expression in cultured HL-1 cells. (A) α-MHC and β-MHC, (B) ANF, (C) α-skeletal actin (α-skeletal), (D) α-cardiac actin (α-cardiac), and (E) connexin43 (C43) in controls and cultured HL-1 cells at passage 86. Total RNA was isolated from AT-1 cells (AT-1), HL-1 cells (HL-1), ventricular cardiac muscle tissue from 17 day embryonic rat (e17), atrial muscle tissue from adult mouse (MA), ventricular cardiac muscle tissue from adult mouse (MV), and skeletal muscle tissue from adult mouse (MS). DNA molecular weight markers [φX174 RF DNA, HaeIII cut] (M).
Electrophysiological Characterization.
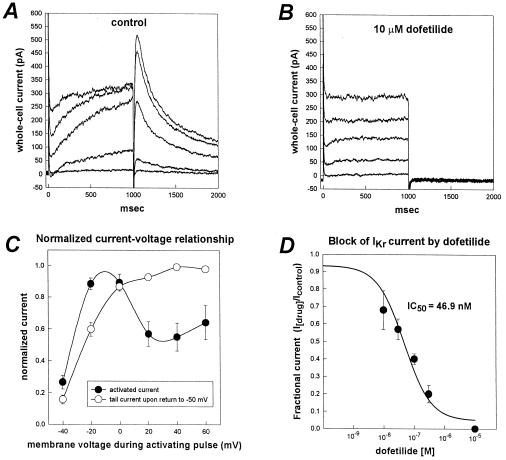
After several days in culture, HL-1 cells exhibited spontaneous action potentials (not shown) and synchronous beating in confluent cultures. We used the whole-cell mode of the patch-clamp technique to identify the repolarizing K+ currents present in HL-1 cells. Depolarizing voltage clamp pulses activated a time-dependent outward current (Fig. 3A). The time-dependent activating current displayed inward rectification (Fig. 3C) for depolarizing pulses positive to 0 mV. Deactivating current tails were voltage-dependent and saturated between +20 mV and +30 mV (Fig. 3 A and C). The deactivation kinetics of the tail current were biexponential. These properties are similar to the current IKr, a delayed rectifier K+ current characteristic of cardiac myocytes. IKr-like current was the most prominent outward current in HL-1 cells and was found in 61 of 65 cells examined. The time-dependent activating currents and deactivating tail currents were highly sensitive to the methanesulfonanilide, dofetilide (Fig. 3 B and D); IC50 of 46.9 nM (n = 4). Such sensitivity to dofetilide identifies this current as cardiac IKr given the high selectivity of this compound for this channel (18). By using the same experimental conditions as described above, except that the holding potential was −70 mV and there was no Cd2+ in the solution, time- and voltage-dependent inward currents also were observed in the HL-1 cells (data not shown). These currents are most likely sodium and L-type calcium currents.
Figure 3.
Characteristics of IKr-like current recorded from HL-1 cells. (A) Whole-cell current traces recorded from an HL-1 cell. Voltage pulses (1-sec duration) were applied from a holding potential of −50 mV to test potentials between −40 mV to +40 mV in 20-mV increments. The membrane potential was returned to −50 mV after each depolarizing pulse to better resolve outward tail currents. (B) Whole-cell currents in the presence of 10 μM of dofetilide (same cell as in A). Dofetilide abolished the time-dependent component of the activating current and the deactivating tail currents. Complete blockade of the time-dependent component of the activating outward current revealed a residual time-independent outward current in most HL-1 cells. (C) Current-voltage relationship for the time-dependent activating outward current and the deactivating tail current. Plotted are the normalized magnitudes of whole-cell currents recorded at the end of each 1-sec depolarizing pulse (•) and tail currents (○) versus membrane potential. Currents were normalized as a fraction of peak current magnitude. Values are means ± SEM (n = 10 cells). (D) Concentration-response relationship for block of IKr tail current by dofetilide. IC50 was 46.9 nM (n = 4).
Dofetilide Binding.
In saturation binding experiments, HL-1 cells at a concentration of 2.4 × 105 cells per ml were incubated with 200, 100, 50, 25, 12.5, or 6.25 nM [3H]dofetilide both in the presence and absence of 20 μM unlabeled dofetilide. The binding was saturable, with a Kd of 140 +/− 60 nM and a Bmax of 118 fmol per 105 cells. The Kd for [3H]dofetilide binding to HL-1 cells is not significantly different from the Kd (70 +/− 6 nM) determined in guinea pig myocytes (19).
In competition binding experiments HL-1 cells (2 to 3 × 105 per ml) from passage 60 to 80 were incubated with 30 to 40 nM [3H]dofetilide and various concentrations of known Ikr blockers, clofilium, azimilide dihydrochloride, and dofetilide. The Kd values determined in HL-1 cells for dofetilide and clofilium are in agreement with the values obtained in [3H]dofetilide binding experiments on guinea pig myocytes (Table 1). The Kd for azimilide dihydrochloride is in agreement with the IC50 (0.4 μM) for blocking the rapidly activating component of the delayed rectifier IKr in ferret papillary muscle (19).
Table 1.
Competition for [3H]dofetilide binding in HL-1 cells and guinea pig myocytes
| Drug | HL-1 cells
|
Cardiac myocytes*
|
||||
|---|---|---|---|---|---|---|
| N | Kd, μM | SEM, μM | N | Kd, μM | SEM, μM | |
| Dofetilide | 4 | 0.042 | 0.02 | 13 | 0.10 | 0.03 |
| Clofilium | 3 | 0.830 | 0.43 | 5 | 1.29 | 0.32 |
| Azimilide | 6 | 0.800 | 0.14 | |||
Values for dofetilide and clofilium obtained in guinea pig ventricular myocytes (16).
DISCUSSION
This report describes an immortal cell line with a phenotype that is similar to adult cardiomyocytes. These cardiac characteristics of HL-1 cells include the following: (i) an ultrastructure similar to primary cultures of adult atrial cardiac myocytes (20) and AT-1 cells (3); (ii) cytoplasmic reorganization and myofibrillogenesis similar to that observed in mitotic cardiomyocytes of the developing heart (17); (iii) presence of highly ordered myofibrils and cardiac-specific junctions in the form of intercalated discs (Fig. 1); (iv) the ability to undergo spontaneous contractions while remaining in a mitotic state typical of normal in vivo immature mitotic cardiomyocytes; (v) expression of ANF, α-MHC, α-cardiac actin, desmin, and connexin43; and (vi) presence of several voltage-dependent currents that are characteristic of a cardiac myocyte phenotype, particularly the IKr current, which has not been identified in noncardiac tissues.
What exactly do HL-1 cells represent? We believe that they represent somewhat of a hybrid between an embryonic and an adult myocyte rather than an intermediate stage of myocyte maturation. By their very nature because they are dividing they have a less differentiated and less organized ultrastructure similar to a mitotic embryonic myocyte. Morphologically they do contain atrial granules however, which is similar to the adult myocyte. They also display an adult myocyte pattern of gene expression for a subset of sarcomeric genes.
HL-1 cells possess important properties not found in their progenitors, the AT-1 cells. The latter maintain a differentiated cardiac phenotype, but they must be carried as a subcutaneous tumor lineage in syngeneic mice because they cannot be passaged in culture. HL-1 cells, in contrast, retain a differentiated phenotype during continuous passage in culture. However, as a consequence of their capacity to continuously divide in culture, they contain fewer highly organized parallel arrays of myofibrils and fewer perinuclear atrial granules than do normal adult atrial cardiomyocytes. This morphological pattern is similar to that of actively dividing embryonic cardiac myocytes.
HL-1 cells differ from other immortalized cardiac cells in their ability to maintain their cardiac phenotype during extended passages. A cardiac muscle cell line named AT-2 cells has been derived from atrial tissue of transgenic mice expressing SV40 large T antigen under control of the α-cardiac MHC promoter (5, 6). AT-2 cells rapidly divide in culture and can be easily passaged but lose their muscle phenotype after about the 10th passage in culture (5). Another cell line, termed MCM1, has been derived from an atrial tumor from a transgenic mouse expressing the SV40 T antigen driven by the protamine 1 promoter. These cells retain many of the differentiated properties of cardiac cells, but they grow very slowly and exhibit a gradual decrease in the percentage of differentiated cells when propagated as a monolayer (7). Attempts by others to produce a cardiac myocyte cell line have resulted in a similar pattern of diminution or loss of phenotype (21).
Quail cardiac myoblasts transformed with v-Myc have been passaged more than 60 times. These cells progressively lose their muscle markers with time in culture although coculture of these cells with 3T3 mouse fibroblasts induces a moderate re-expression of muscle myosin (11). However, localization of this protein is diffuse, and the cells do not form organized sarcomeres and do not contract. Similar results on the establishment of a cell line (QCE-6) from precardiac splanchnic mesoderm of the Japanese quail embryo recently were reported (13). During the establishment of this cell line, muscle-specific phenotypic markers were lost. The addition of retinoic acid and growth factors induced cardiomyogenic differentiation in approximately 50% of the QCE-6 cells as indicated by the expression of cardiac-specific proteins (13). However, neither myofibrils nor contractile activity was detected in these cultured cells. Nevertheless, the QCE-6 cells may prove to be useful for the study of cardiac muscle differentiation because they appear to be progenitors of the cardiac myogenic cell lineage.
Embryonic rat cardiac myocytes transfected with the v-Myc and v-H-Ras oncogenes have been reported to undergo multiple passages in culture (12). These cells retain the expression of several cardiac-specific genes but have no organized ultrastructure and do not contract (12). Human fetal cardiac muscle cells transfected with SV40 T antigen can be maintained for over a year in culture (9). These cells expressed several markers of early fetal human cardiac myocytes but did not contract (9). Two other reports (8, 10) also have established that the expression of SV40 T antigen in neonatal rat cardiomyocytes induces myocyte proliferation. However, the resulting cells either lost the ability to proliferate or lost most of their highly differentiated phenotype.
The HL-1 cells retain a pattern of gene expression characteristic of normal adult mouse myocytes (Fig. 2). They express genes coding for adult protein isoforms (α-MHC, α-cardiac actin) despite the fact that they are actively dividing. In contrast, fetal and neonatal cardiomyocytes express the embryonic isoforms, i.e., β-MHC and α-skeletal actin (22, 23). HL-1 cardiomyocytes therefore are unique in their ability to proliferate and to be repeatedly passaged without reverting to an embryonic phenotype. These properties make HL-1 cells particularly attractive for studies that require expression of adult-specific genes and for studies of cardiomyocyte function. In addition, this cellular model will be useful for assessing the activity of pharmacological agents such as modulators of cardiac ion channels.
Acknowledgments
We thank Carol Thouron and Cathy Vial for help with the confocal and transmission electron microscopy and Michael W. Scherz and Paul M. Dybas for the synthesis and analysis, respectively, of [3H]dofetilide. This research was supported by National Institutes of Health Grant HL 43124.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ANF, atrial natriuretic factor; SV40, simian virus 40; MHC, myosin heavy chain.
References
- 1.Field L J. Science. 1988;239:1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- 2.Steinhelper M E, Lanson N A, Jr, Dresdner K P, Delcarpio J B, Wit A L, Claycomb W C, Field L J. Am J Physiol. 1990;259:H1826–H1834. doi: 10.1152/ajpheart.1990.259.6.H1826. [DOI] [PubMed] [Google Scholar]
- 3.Delcarpio J B, Lanson N A, Jr, Field L J, Claycomb W C. Circ Res. 1991;69:1591–1600. doi: 10.1161/01.res.69.6.1591. [DOI] [PubMed] [Google Scholar]
- 4.Lanson N A, Jr, Glembotski C C, Steinhelper M E, Field L J, Claycomb W C. Circulation. 1992;85:1835–1841. doi: 10.1161/01.cir.85.5.1835. [DOI] [PubMed] [Google Scholar]
- 5.Borisov A B, Claycomb W C. Ann NY Acad Sci. 1995;752:80–91. doi: 10.1111/j.1749-6632.1995.tb17408.x. [DOI] [PubMed] [Google Scholar]
- 6.Katz E B, Steinhelper M E, Delcarpio J B, Daud A I, Claycomb W C, Field L J. Am J Physiol. 1992;262:H1867–H1876. doi: 10.1152/ajpheart.1992.262.6.H1867. [DOI] [PubMed] [Google Scholar]
- 7.Gartside C L, Hauschka S D. In: The Development and Regenerative Potential of Cardiac Muscle. Oberpriller J C, Oberpriller J O, Mauro A, editors. New York: Harwood; 1991. pp. 7941–7948. [Google Scholar]
- 8.Jahn L, Sadoshima J, Greene A, Parker C, Morgan K G, Izumo S. J Cell Sci. 1996;109:397–407. doi: 10.1242/jcs.109.2.397. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y-C, Neckelmann N, Mayne A, Herskowitz A, Srinivasan A, Sell K W, Ahmed-Ansari A. In Vitro Cell Dev Biol. 1991;27:63–74. doi: 10.1007/BF02630896. [DOI] [PubMed] [Google Scholar]
- 10.Sen A, Dunnmon P, Henderson S A, Gerard R D, Chien K R. J Biol Chem. 1988;263:19132–19136. [PubMed] [Google Scholar]
- 11.Jaffredo T, Chestier A, Bachnou N, Dieterlen-Lievre F. Exp Cell Res. 1991;192:481–491. doi: 10.1016/0014-4827(91)90067-5. [DOI] [PubMed] [Google Scholar]
- 12.Engelmann G L, Birchenall-Roberts M C, Ruscetti F W, Samarel A M. J Mol Cell Cardiol. 1993;25:197–213. doi: 10.1006/jmcc.1993.1022. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg C A, Bader D M. Circ Res. 1996;78:205–216. doi: 10.1161/01.res.78.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Delcarpio J B, Claycomb W C, Moses R L. Am J Anat. 1989;186:335–345. doi: 10.1002/aja.1001860403. [DOI] [PubMed] [Google Scholar]
- 15.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Chadwick C C, Ezrin A M, O’Connor B, Volberg W A, Smith D L, Wedge K J, Hill R J, Briggs G M, Pagani E D, Silver P J, Krafte D S. Circ Res. 1993;72:707–714. doi: 10.1161/01.res.72.3.707. [DOI] [PubMed] [Google Scholar]
- 17.Markwald R R. J Mol Cell Cardiol. 1973;5:341–350. doi: 10.1016/0022-2828(73)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Jurkiewicz N K, Sanguinetti M C. Circ Res. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Fermini B, Jurkiewicz N K, Jow B, Guinosso P J, Jr, Baskin E P, Lynch J J, Jr, Salata J J. J Cardiovasc Pharmacol. 1995;26:259–271. doi: 10.1097/00005344-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Moses R L, Claycomb W C. Am J Anat. 1984;171:191–206. doi: 10.1002/aja.1001710205. [DOI] [PubMed] [Google Scholar]
- 21.Kimes B W, Brandt B L. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 22.Sassoon D A, Garner I, Buckingham M. Development (Cambridge, UK) 1988;104:155–164. doi: 10.1242/dev.104.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Lompre A M, Mercadier J J, Wisnewsky C, Bouveret P, Pantaloni C, D’Albis A, Schwartz K. Dev Biol. 1981;84:286–290. doi: 10.1016/0012-1606(81)90396-1. [DOI] [PubMed] [Google Scholar]