Abstract
To understand the role of restriction in regulating gene flow in bacterial populations, we would like to understand the regulation of restriction enzyme activity. Several antirestriction (restriction alleviation) systems are known that reduce the activity of type I restriction enzymes like EcoKI in vivo. Most of these do not act on type II or type III enzymes, but little information is available for the unclassified modification-dependent systems, of which there are three in E. coli K-12. Of particular interest are two physiological controls on type I enzymes: EcoKI restriction is reduced 2 to 3 orders of magnitude following DNA damage, and a similar effect is seen constitutively in Dam- cells. We used the behavior of EcoKI as a control for testing the response to UV treatment of the three endogenous modification-dependent restriction systems of K-12, McrA, McrBC, and Mrr. Two of these were also tested for response to Dam status. We find that all four resident restriction systems show reduced activity following UV treatment, but not in a unified fashion; each response was genetically and physiologically distinct. Possible mechanisms are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barras F., Marinus M. G. The great GATC: DNA methylation in E. coli. Trends Genet. 1989 May;5(5):139–143. doi: 10.1016/0168-9525(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Belogurov A. A., Yussifov T. N., Kotova V. U., Zavilgelsky G. B. The novel gene(s) ARD of plasmid pKM101: alleviation of EcoK restriction. Mol Gen Genet. 1985;198(3):509–513. doi: 10.1007/BF00332948. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Krüger D. H. Biology of DNA restriction. Microbiol Rev. 1993 Jun;57(2):434–450. doi: 10.1128/mr.57.2.434-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell. 1990 Sep 7;62(5):981–989. doi: 10.1016/0092-8674(90)90272-g. [DOI] [PubMed] [Google Scholar]
- Brody H., Greener A., Hill C. W. Excision and reintegration of the Escherichia coli K-12 chromosomal element e14. J Bacteriol. 1985 Mar;161(3):1112–1117. doi: 10.1128/jb.161.3.1112-1117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Davison J. Restriction insensitivity in bacteriophage T5. III. Characterization of EcoRI-sensitive mutants by restriction analysis. J Mol Biol. 1979 Mar 15;128(4):527–543. doi: 10.1016/0022-2836(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Chaudhury A. M., Smith G. R. Role of Escherichia coli RecBC enzyme in SOS induction. Mol Gen Genet. 1985;201(3):525–528. doi: 10.1007/BF00331350. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd UV-induced alleviation of K-specific restriction of bacteriophage lambda. J Virol. 1977 Mar;21(3):1249–1251. doi: 10.1128/jvi.21.3.1249-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoret R., Pierre M., Moreau P. L. Prophage phi 80 is induced in Escherichia coli K12 recA430. Mol Gen Genet. 1983;189(2):199–206. doi: 10.1007/BF00337804. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Goldberg E. B. Mechanism localisation and control of restriction cleavage of phage T4 and lambda chromosomes in vivo. Nature. 1976 Apr 1;260(5550):406–410. doi: 10.1038/260406a0. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Goldberg E. B. Phage-coded protein prevents restriction of unmodified progeny T4 DNA. Nature. 1976 Apr 1;260(5550):454–456. doi: 10.1038/260454a0. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Goldberg E. B. Restriction in vivo. V. Introduction of SOS functions in Escherichia coli by restricted T4 phage DNA, and alleviation of restriction by SOS functions. Mol Gen Genet. 1980 Apr;178(1):51–58. doi: 10.1007/BF00267212. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Revel H. R., Goldberg E. B. Physical mapping and cloning of bacteriophage T4 anti-restriction endonuclease gene. J Bacteriol. 1982 Feb;149(2):694–699. doi: 10.1128/jb.149.2.694-699.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dila D., Sutherland E., Moran L., Slatko B., Raleigh E. A. Genetic and sequence organization of the mcrBC locus of Escherichia coli K-12. J Bacteriol. 1990 Sep;172(9):4888–4900. doi: 10.1128/jb.172.9.4888-4900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova E. P., Delver E. P., Belogurov A. A. 2-Aminopurine and 5-bromouracil induce alleviation of type I restriction in Escherichia coli: mismatches function as inducing signals? Mol Gen Genet. 1988 Oct;214(2):317–320. doi: 10.1007/BF00337728. [DOI] [PubMed] [Google Scholar]
- Efimova E. P., Delver E. P., Belogurov A. A. Alleviation of type I restriction in adenine methylase (dam) mutants of Escherichia coli. Mol Gen Genet. 1988 Oct;214(2):313–316. doi: 10.1007/BF00337727. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. The muc genes of pKM101 are induced by DNA damage. J Bacteriol. 1983 Sep;155(3):1306–1315. doi: 10.1128/jb.155.3.1306-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endlich B., Linn S. The DNA restriction endonuclease of Escherichia coli B. II. Further studies of the structure of DNA intermediates and products. J Biol Chem. 1985 May 10;260(9):5729–5738. [PubMed] [Google Scholar]
- Ennis D. G., Ossanna N., Mount D. W. Genetic separation of Escherichia coli recA functions for SOS mutagenesis and repressor cleavage. J Bacteriol. 1989 May;171(5):2533–2541. doi: 10.1128/jb.171.5.2533-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOVER S. W., COLSON C. THE BREAKDOWN OF THE RESTRICTION MECHANISM IN ZYGOTES OF ESCHERICHIA COLI. Genet Res. 1965 Feb;6:153–155. doi: 10.1017/s001667230000402x. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heip J., Rolfe B., Schell J. Abolition of host cell restriction by high multiplicity of phage infection. Virology. 1974 Jun;59(2):356–370. doi: 10.1016/0042-6822(74)90450-4. [DOI] [PubMed] [Google Scholar]
- Heitman J., Model P. Site-specific methylases induce the SOS DNA repair response in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3243–3250. doi: 10.1128/jb.169.7.3243-3250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Zinder N. D., Model P. Repair of the Escherichia coli chromosome after in vivo scission by the EcoRI endonuclease. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2281–2285. doi: 10.1073/pnas.86.7.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiom K. J., Sedgwick S. G. Alleviation of EcoK DNA restriction in Escherichia coli and involvement of umuDC activity. Mol Gen Genet. 1992 Jan;231(2):265–275. doi: 10.1007/BF00279800. [DOI] [PubMed] [Google Scholar]
- Hiom K., Sedgwick S. G. Cloning and structural characterization of the mcrA locus of Escherichia coli. J Bacteriol. 1991 Nov;173(22):7368–7373. doi: 10.1128/jb.173.22.7368-7373.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. L., Nuttall S. D., Dyall-Smith M. L. Construction and use of halobacterial shuttle vectors and further studies on Haloferax DNA gyrase. J Bacteriol. 1991 Jun;173(12):3807–3813. doi: 10.1128/jb.173.12.3807-3813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Iida S., Streiff M. B., Bickle T. A., Arber W. Two DNA antirestriction systems of bacteriophage P1, darA, and darB: characterization of darA- phages. Virology. 1987 Mar;157(1):156–166. doi: 10.1016/0042-6822(87)90324-2. [DOI] [PubMed] [Google Scholar]
- Kaiser K., Murray N. E. Physical characterisation of the "Rac prophage" in E. coli K12. Mol Gen Genet. 1979 Sep;175(2):159–174. doi: 10.1007/BF00425532. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Kelleher J. E., Daniel A. S., Murray N. E. Mutations that confer de novo activity upon a maintenance methyltransferase. J Mol Biol. 1991 Sep 20;221(2):431–440. doi: 10.1016/0022-2836(91)80064-2. [DOI] [PubMed] [Google Scholar]
- Kelleher J. E., Raleigh E. A. A novel activity in Escherichia coli K-12 that directs restriction of DNA modified at CG dinucleotides. J Bacteriol. 1991 Aug;173(16):5220–5223. doi: 10.1128/jb.173.16.5220-5223.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukalová B., Kuhrová V., Reich J. Protection of nonmodified phage lambda against EcoK restriction mediated by recA protein. Folia Microbiol (Praha) 1985;30(1):17–24. doi: 10.1007/BF02922492. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. The SOS regulatory system: control of its state by the level of RecA protease. J Mol Biol. 1983 Jul 15;167(4):791–808. doi: 10.1016/s0022-2836(83)80111-9. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Buckman C. Identification and genetic analysis of sbcC mutations in commonly used recBC sbcB strains of Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):836–844. doi: 10.1128/jb.164.2.836-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Daniel A. S., Braymer H. D., Murray N. E. Organization and sequence of the hsd genes of Escherichia coli K-12. J Mol Biol. 1987 Nov 20;198(2):159–170. doi: 10.1016/0022-2836(87)90303-2. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Murray N. E. Modification enhancement by the restriction alleviation protein (Ral) of bacteriophage lambda. J Mol Biol. 1986 Jul 5;190(1):11–22. doi: 10.1016/0022-2836(86)90071-9. [DOI] [PubMed] [Google Scholar]
- MacNeil D. J. Characterization of a unique methyl-specific restriction system in Streptomyces avermitilis. J Bacteriol. 1988 Dec;170(12):5607–5612. doi: 10.1128/jb.170.12.5607-5612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso A., Mettus A. M. Efficient transformation of Bacillus thuringiensis requires nonmethylated plasmid DNA. J Bacteriol. 1991 Feb;173(3):1353–1356. doi: 10.1128/jb.173.3.1353-1356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G. DNA methylation influences trpR promoter activity in Escherichia coli K-12. Mol Gen Genet. 1985;200(1):185–186. doi: 10.1007/BF00383334. [DOI] [PubMed] [Google Scholar]
- McPartland A., Green L., Echols H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell. 1980 Jul;20(3):731–737. doi: 10.1016/0092-8674(80)90319-0. [DOI] [PubMed] [Google Scholar]
- Peterson K. R., Mount D. W. Analysis of the genetic requirements for viability of Escherichia coli K-12 DNA adenine methylase (dam) mutants. J Bacteriol. 1993 Nov;175(22):7505–7508. doi: 10.1128/jb.175.22.7505-7508.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Wertman K. F., Mount D. W., Marinus M. G. Viability of Escherichia coli K-12 DNA adenine methylase (dam) mutants requires increased expression of specific genes in the SOS regulon. Mol Gen Genet. 1985;201(1):14–19. doi: 10.1007/BF00397979. [DOI] [PubMed] [Google Scholar]
- Price C., Bickle T. A. A possible role for DNA restriction in bacterial evolution. Microbiol Sci. 1986 Oct;3(10):296–299. [PubMed] [Google Scholar]
- Raleigh E. A., Benner J., Bloom F., Braymer H. D., DeCruz E., Dharmalingam K., Heitman J., Noyer Weidner M., Piekarowicz A., Kretz P. L. Nomenclature relating to restriction of modified DNA in Escherichia coli. J Bacteriol. 1991 Apr;173(8):2707–2709. doi: 10.1128/jb.173.8.2707-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A. Organization and function of the mcrBC genes of Escherichia coli K-12. Mol Microbiol. 1992 May;6(9):1079–1086. doi: 10.1111/j.1365-2958.1992.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Trimarchi R., Revel H. Genetic and physical mapping of the mcrA (rglA) and mcrB (rglB) loci of Escherichia coli K-12. Genetics. 1989 Jun;122(2):279–296. doi: 10.1093/genetics/122.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Wilson G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9070–9074. doi: 10.1073/pnas.83.23.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read T. D., Thomas A. T., Wilkins B. M. Evasion of type I and type II DNA restriction systems by IncI1 plasmid CoIIb-P9 during transfer by bacterial conjugation. Mol Microbiol. 1992 Jul;6(14):1933–1941. doi: 10.1111/j.1365-2958.1992.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Ream L. W., Margossian L., Clark A. J., Hansen F. G., von Meyenburg K. Genetic and physical mapping of recF in Escherichia coli K-12. Mol Gen Genet. 1980;180(1):115–121. doi: 10.1007/BF00267359. [DOI] [PubMed] [Google Scholar]
- Roberts D., Hoopes B. C., McClure W. R., Kleckner N. IS10 transposition is regulated by DNA adenine methylation. Cell. 1985 Nov;43(1):117–130. doi: 10.1016/0092-8674(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Two mutations that alter the regulatory activity of E. coli recA protein. Nature. 1981 Apr 2;290(5805):422–424. doi: 10.1038/290422a0. [DOI] [PubMed] [Google Scholar]
- Sassanfar M., Roberts J. W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J Mol Biol. 1990 Mar 5;212(1):79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Yarranton G. T., Heath R. W. Lysogenic induction of lambdoid phages in lexA mutants of Escherichia coli. Mol Gen Genet. 1981;184(3):457–459. doi: 10.1007/BF00352522. [DOI] [PubMed] [Google Scholar]
- Simmon V. F., Lederberg S. Degradation of bacteriophage lambda deoxyribonucleic acid after restriction by Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):161–169. doi: 10.1128/jb.112.1.161-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Nowak J. A., Maniloff J. Mycoplasma restriction: identification of a new type of restriction specificity for DNA containing 5-methylcytosine. J Bacteriol. 1986 Jan;165(1):219–225. doi: 10.1128/jb.165.1.219-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerel N., Herrlich P., Bickle T. A. A novel bacteriophage defence mechanism: the anti-restriction protein. Nature. 1979 Mar 1;278(5699):30–34. doi: 10.1038/278030a0. [DOI] [PubMed] [Google Scholar]
- Sutherland E., Coe L., Raleigh E. A. McrBC: a multisubunit GTP-dependent restriction endonuclease. J Mol Biol. 1992 May 20;225(2):327–348. doi: 10.1016/0022-2836(92)90925-a. [DOI] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Expression of ultraviolet-induced restriction alleviation in Escherichia coli K-12. Detection of a lambda phage fraction with a retarded mode of DNA injection. Biochim Biophys Acta. 1983 Jan 20;739(1):42–47. doi: 10.1016/0167-4781(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Genetic control of damage-inducible restriction alleviation in Escherichia coli K12: an SOS function not repressed by lexA. Mol Gen Genet. 1984;197(2):297–303. doi: 10.1007/BF00330977. [DOI] [PubMed] [Google Scholar]
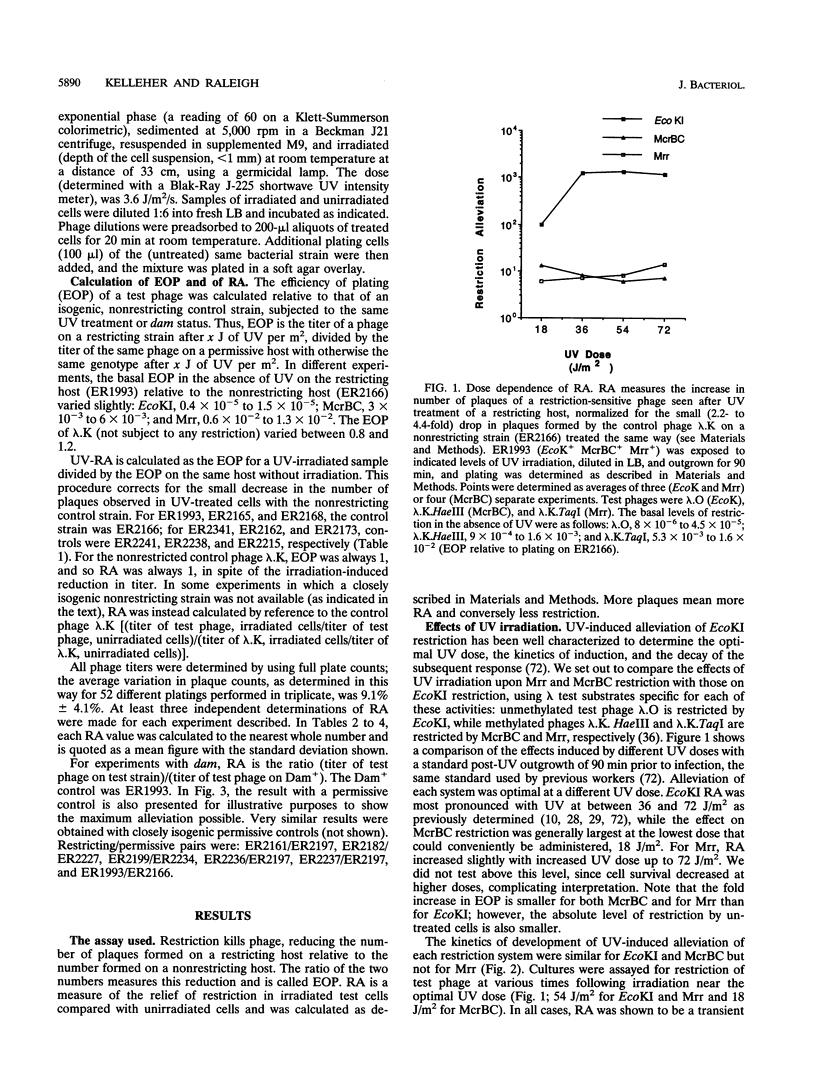
- Thoms B., Wackernagel W. UV-induced allevation of lambda restriction in Escherichia coli K-12: kinetics of induction and specificity of this SOS function. Mol Gen Genet. 1982;186(1):111–117. doi: 10.1007/BF00422921. [DOI] [PubMed] [Google Scholar]
- Toothman P. Restriction alleviation by bacteriophages lambda and lambda reverse. J Virol. 1981 May;38(2):621–631. doi: 10.1128/jvi.38.2.621-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertès A. A., Inui M., Kobayashi M., Kurusu Y., Yukawa H. Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res Microbiol. 1993 Mar-Apr;144(3):181–185. doi: 10.1016/0923-2508(93)90043-2. [DOI] [PubMed] [Google Scholar]
- Waite-Rees P. A., Keating C. J., Moran L. S., Slatko B. E., Hornstra L. J., Benner J. S. Characterization and expression of the Escherichia coli Mrr restriction system. J Bacteriol. 1991 Aug;173(16):5207–5219. doi: 10.1128/jb.173.16.5207-5219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Smith K. C. Inviability of dam recA and dam recB cells of Escherichia coli is correlated with their inability to repair DNA double-strand breaks produced by mismatch repair. J Bacteriol. 1986 Mar;165(3):1023–1025. doi: 10.1128/jb.165.3.1023-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiserova M., Janscak P., Benada O., Hubácek J., Zinkevich V. E., Glover S. W., Firman K. Cloning, production and characterisation of wild type and mutant forms of the R.EcoK endonucleases. Nucleic Acids Res. 1993 Feb 11;21(3):373–379. doi: 10.1093/nar/21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Murray N. E. Restriction and modification systems. Annu Rev Genet. 1991;25:585–627. doi: 10.1146/annurev.ge.25.120191.003101. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Friedman S., Van Montagu M., Schell J. The ral gene of phage lambda. I. Identification of a non-essential gene that modulates restriction and modification in E. coli. Mol Gen Genet. 1980;179(1):63–73. doi: 10.1007/BF00268447. [DOI] [PubMed] [Google Scholar]



