Abstract
A ferulic acid decarboxylase enzyme which catalyzes the decarboxylation of ferulic acid to 4-hydroxy-3-methoxystyrene was purified from Pseudomonas fluorescens UI 670. The enzyme requires no cofactors and contains no prosthetic groups. Gel filtration estimated an apparent molecular mass of 40.4 (+/- 6%) kDa, whereas sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed a molecular mass of 20.4 kDa, indicating that ferulic acid decarboxylase is a homodimer in solution. The purified enzyme displayed an optimum temperature range of 27 to 30 degrees C, exhibited an optimum pH of 7.3 in potassium phosphate buffer, and had a Km of 7.9 mM for ferulic acid. This enzyme also decarboxylated 4-hydroxycinnamic acid but not 2- or 3-hydroxycinnamic acid, indicating that a hydroxy group para to the carboxylic acid-containing side chain is required for the enzymatic reaction. The enzyme was inactivated by Hg2+, Cu2+, p-chloromercuribenzoic acid, and N-ethylmaleimide, suggesting that sulfhydryl groups are necessary for enzyme activity. Diethyl pyrocarbonate, a histidine-specific inhibitor, did not affect enzyme activity.
Full text
PDF
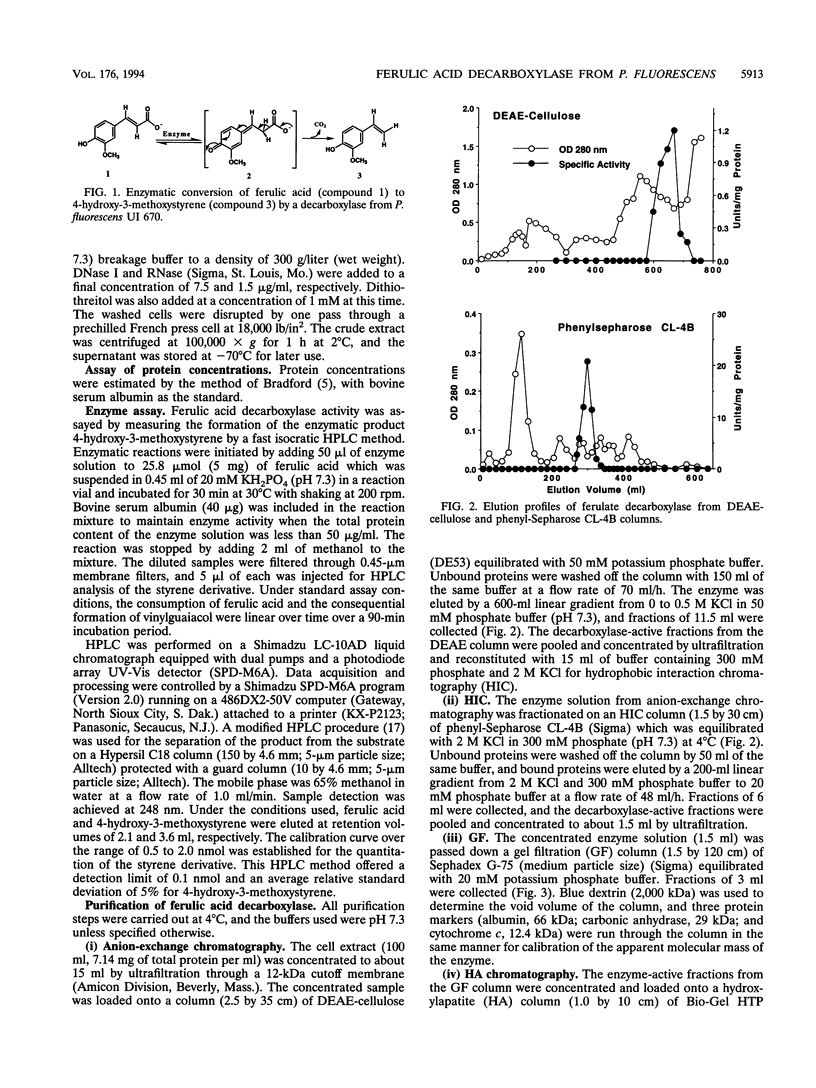
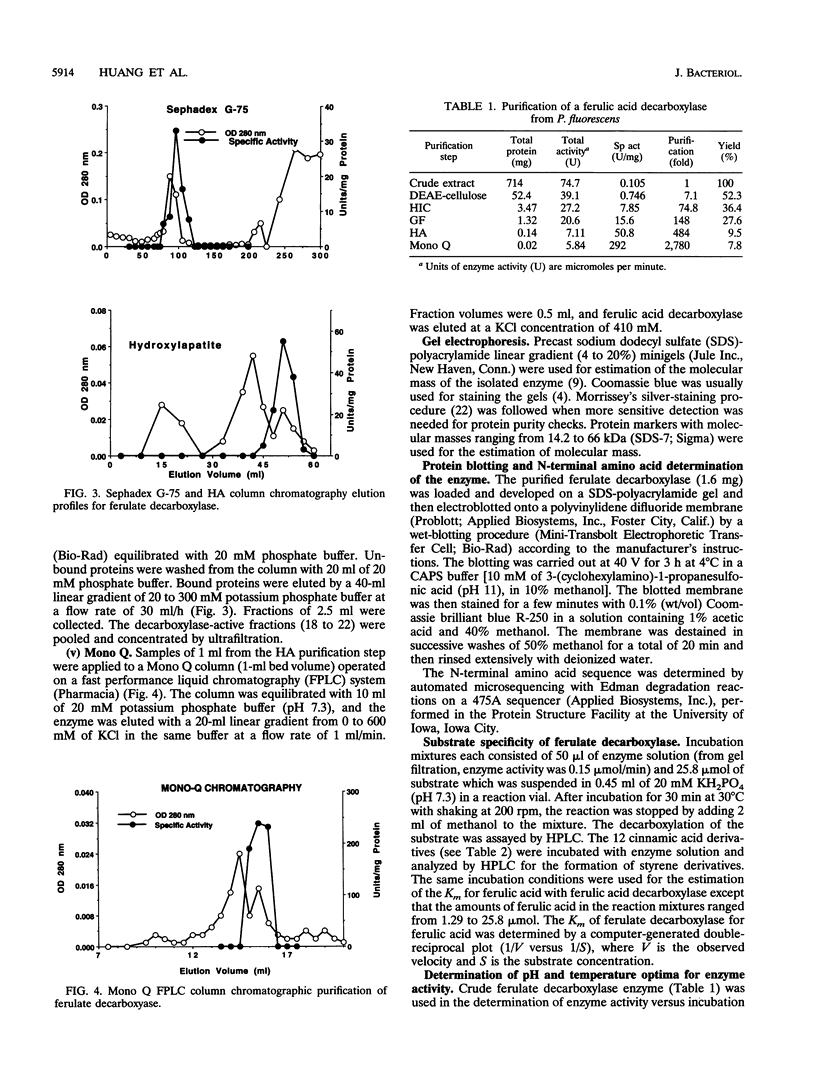
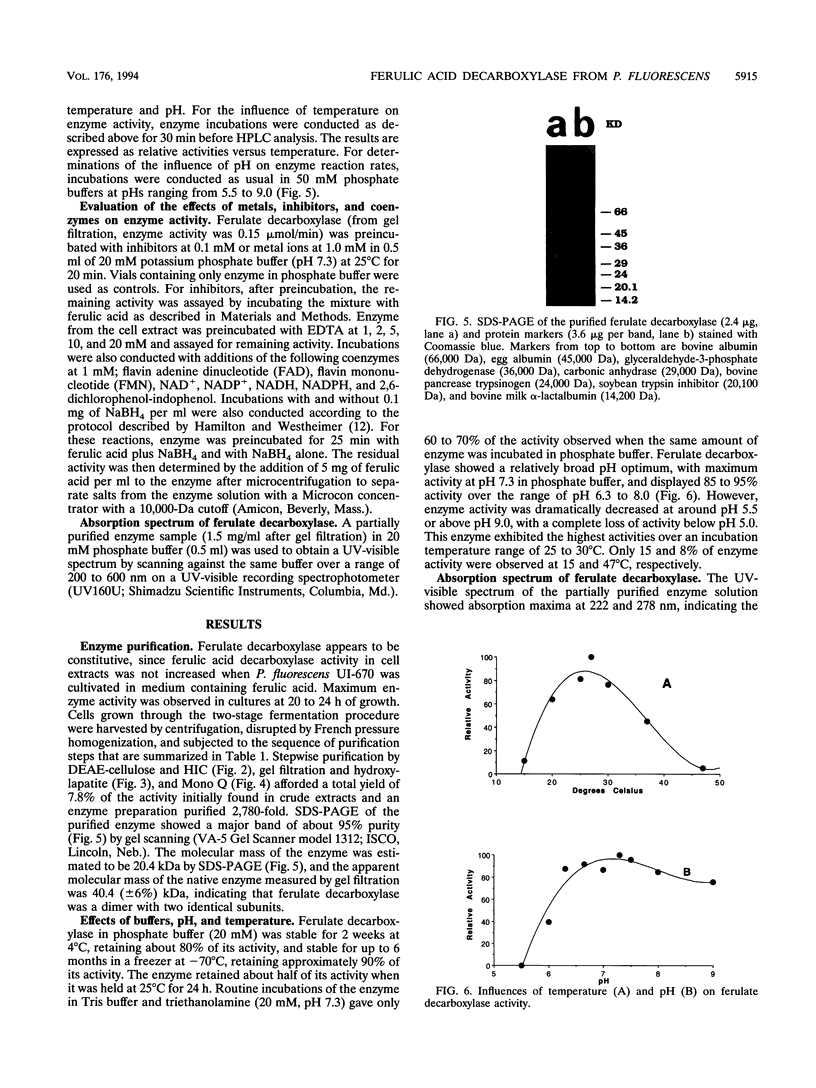



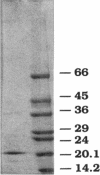
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buswell J. A., Ander P., Pettersson B., Eriksson K. E. Oxidative decarboxylation of vanillic acid by Sporotrichum pulverulentum. FEBS Lett. 1979 Jul 1;103(1):98–101. doi: 10.1016/0014-5793(79)81258-2. [DOI] [PubMed] [Google Scholar]
- Dominici P., Tancini B., Borri Voltattorni C. Chemical modification of pig kidney 3,4-dihydroxyphenylalanine decarboxylase with diethyl pyrocarbonate. Evidence for an essential histidyl residue. J Biol Chem. 1985 Sep 5;260(19):10583–10589. [PubMed] [Google Scholar]
- FINKLE B. J., LEWIS J. C., CORSE J. W., LUNDIN R. E. Enzyme reactions with phenolic compounds: formation of hydroxystyrenes through the decarboxylation of 4-hydroxycinnamic acids by Aerobacter. J Biol Chem. 1962 Sep;237:2926–2931. [PubMed] [Google Scholar]
- Harada T., Mino Y. Some properties of p-coumarate decarboxylase from Cladosporium phlei. Can J Microbiol. 1976 Sep;22(9):1258–1262. doi: 10.1139/m76-186. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. The purification and properties of p-cresol-(acceptor) oxidoreductase (hydroxylating), a flavocytochrome from Pseudomonas putida. Biochem J. 1977 Oct 1;167(1):155–162. doi: 10.1042/bj1670155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. The hydroxylation of P-cresol and its conversion to P-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976 Mar 22;69(2):462–468. doi: 10.1016/0006-291x(76)90544-1. [DOI] [PubMed] [Google Scholar]
- Huang Z., Dostal L., Rosazza J. P. Mechanisms of ferulic acid conversions to vanillic acid and guaiacol by Rhodotorula rubra. J Biol Chem. 1993 Nov 15;268(32):23954–23958. [PubMed] [Google Scholar]
- Huang Z., Dostal L., Rosazza J. P. Microbial transformations of ferulic acid by Saccharomyces cerevisiae and Pseudomonas fluorescens. Appl Environ Microbiol. 1993 Jul;59(7):2244–2250. doi: 10.1128/aem.59.7.2244-2250.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Craig J. C., Everhart E. T., Webster R. V., Causer M. J., Singer T. P. Stereochemistry of 1-(4'-hydroxyphenyl)ethanol produced by hydroxylation of 4-ethylphenol by p-cresol methylhydroxylase. Biochem J. 1984 Dec 1;224(2):617–621. doi: 10.1042/bj2240617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Rahouti M., Seigle-Murandi F., Steiman R., Eriksson K. E. Metabolism of Ferulic Acid by Paecilomyces variotii and Pestalotia palmarum. Appl Environ Microbiol. 1989 Sep;55(9):2391–2398. doi: 10.1128/aem.55.9.2391-2398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Hutchinson C. R. Tetracenomycin F1 monooxygenase: oxidation of a naphthacenone to a naphthacenequinone in the biosynthesis of tetracenomycin C in Streptomyces glaucescens. Biochemistry. 1993 Jul 6;32(26):6656–6663. doi: 10.1021/bi00077a019. [DOI] [PubMed] [Google Scholar]
- Whited G. M., Gibson D. T. Separation and partial characterization of the enzymes of the toluene-4-monooxygenase catabolic pathway in Pseudomonas mendocina KR1. J Bacteriol. 1991 May;173(9):3017–3020. doi: 10.1128/jb.173.9.3017-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E., van Berkel W. J., van der Zwan R. P., de Bont J. A. Purification and characterization of vanillyl-alcohol oxidase from Penicillium simplicissimum. A novel aromatic alcohol oxidase containing covalently bound FAD. Eur J Biochem. 1992 Sep 15;208(3):651–657. doi: 10.1111/j.1432-1033.1992.tb17231.x. [DOI] [PubMed] [Google Scholar]