Abstract
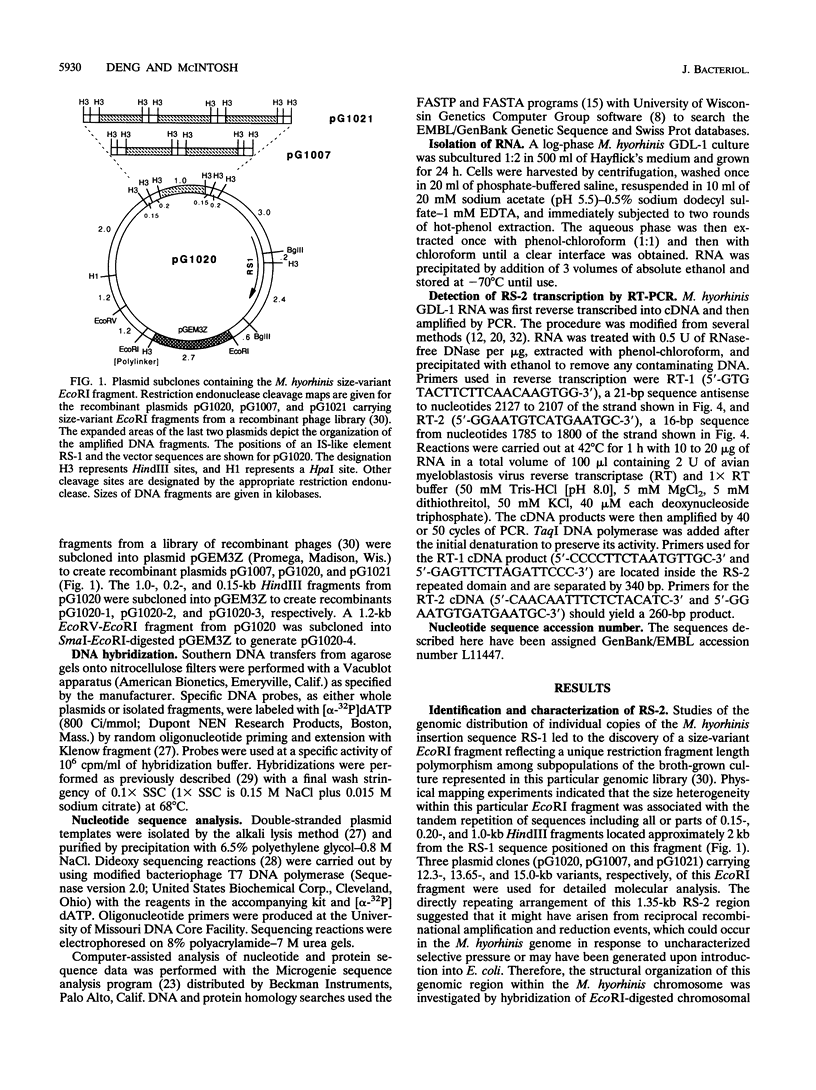
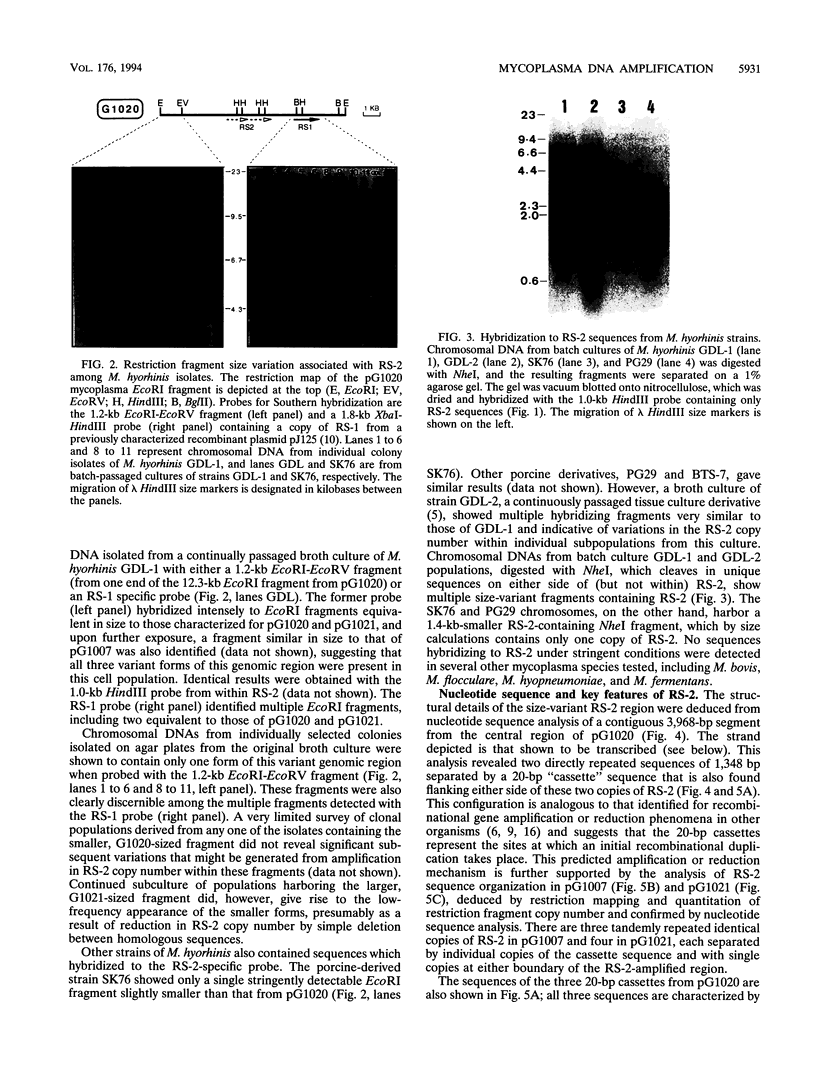
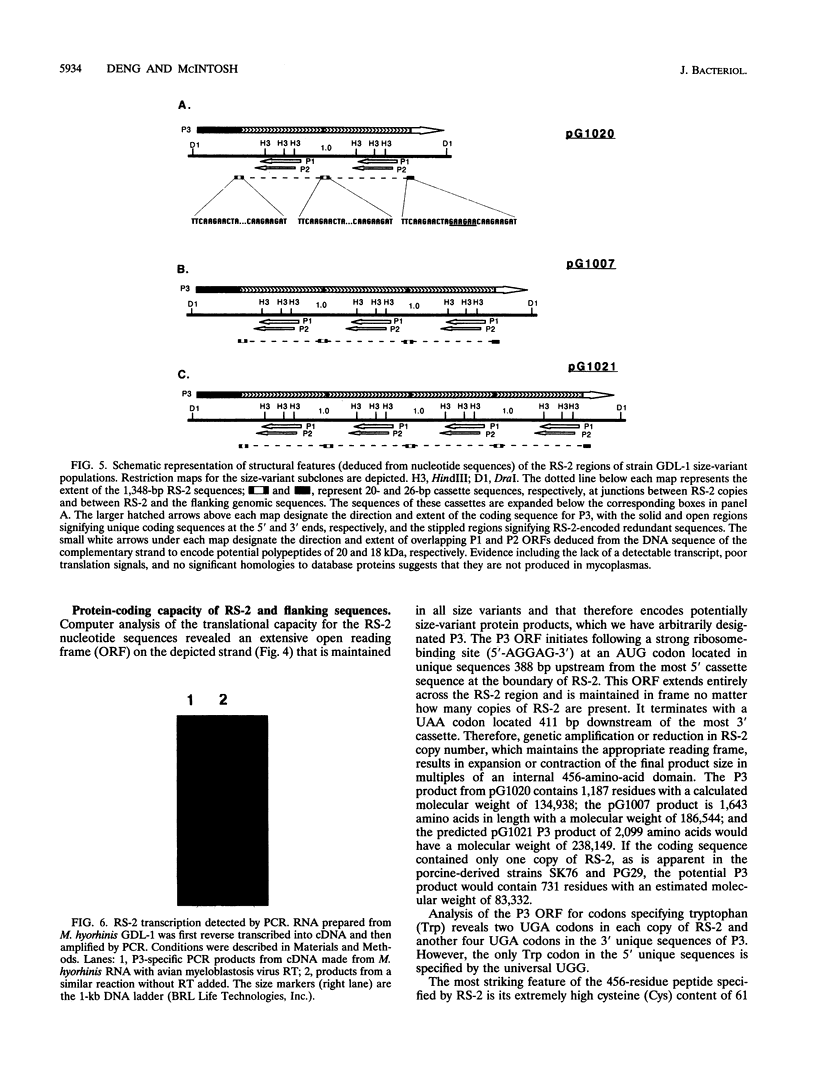
A novel amplifiable genomic region that displays variability in the number of tandem copies of a 1,368-bp DNA sequence (designated RS-2) was discovered among individual clonal derivatives within Mycoplasma hyorhinis broth-grown cell populations. Clonal isolates representing variant subpopulations from the original broth culture were of a single size variant, and although continued culture under a variety of growth conditions did not result in further amplification of RS-2, evidence for deletion events which reduced RS-2 copy number, presumably by homologous recombination, was obtained. RS-2 homologous sequences were identified in all M. hyorhinis strains tested, but only the tissue culture-derived strains GDL-1 and GDL-2 showed variability in genomic dosage. The RS-2 nucleotide sequence established that each tandem copy is flanked by direct repeats of a 20-bp sequence and suggested a possible mechanism for its original duplication as the initial phase of a genetic amplification process. The coding strand was defined by PCR amplification of a reverse transcriptase-generated cDNA, and its sequence revealed that RS-2 encodes a 456-residue internal, highly cysteine-rich domain of a larger M. hyorhinis protein whose coding sequence initiates and terminates in unique genomic sequences several hundred base pairs from RS-2 on either side of it. Changes in RS-2 copy number maintain the reading frame, and therefore the coding capacity, for this predicted size-variant protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Bhugra B., Dybvig K. Identification and characterization of IS1138, a transposable element from Mycoplasma pulmonis that belongs to the IS3 family. Mol Microbiol. 1993 Feb;7(4):577–584. doi: 10.1111/j.1365-2958.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Renkawitz R. Repetitive sequence involvement in the duplication and divergence of mouse lysozyme genes. EMBO J. 1990 Apr;9(4):1283–1288. doi: 10.1002/j.1460-2075.1990.tb08237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Normark S. Recombination between short DNA homologies causes tandem duplication. Nature. 1981 Jul 16;292(5820):269–271. doi: 10.1038/292269a0. [DOI] [PubMed] [Google Scholar]
- Ferrell R. V., Heidari M. B., Wise K. S., McIntosh M. A. A Mycoplasma genetic element resembling prokaryotic insertion sequences. Mol Microbiol. 1989 Jul;3(7):957–967. doi: 10.1111/j.1365-2958.1989.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., McGuire W. L. A simple polymerase chain reaction method for detection and cloning of low-abundance transcripts. Biotechniques. 1990 Aug;9(2):206–211. [PubMed] [Google Scholar]
- Hu W. S., Wang R. Y., Liou R. S., Shih J. W., Lo S. C. Identification of an insertion-sequence-like genetic element in the newly recognized human pathogen Mycoplasma incognitus. Gene. 1990 Sep 1;93(1):67–72. doi: 10.1016/0378-1119(90)90137-g. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maeda N., Smithies O. The evolution of multigene families: human haptoglobin genes. Annu Rev Genet. 1986;20:81–108. doi: 10.1146/annurev.ge.20.120186.000501. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Mesas J. M., Noirot P., Morel-Deville F., Ehrlich S. D. Induction of DNA amplification in the Bacillus subtilis chromosome. EMBO J. 1992 Apr;11(4):1317–1326. doi: 10.1002/j.1460-2075.1992.tb05176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., Ferrell R. V., Wise K. S., McIntosh M. A. Reiterated DNA sequences defining genomic diversity within the species Mycoplasma hyorhinis. Mol Microbiol. 1988 Sep;2(5):665–672. doi: 10.1111/j.1365-2958.1988.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., McIntosh M. A., Robbins J., Wise K. S. Cloned genomic DNA sequences from Mycoplasma hyorhinis encoding antigens expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4154–4158. doi: 10.1073/pnas.80.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., Wise K. S., McIntosh M. A. Selective detection of Mycoplasma hyorhinis using cloned genomic DNA fragments. Infect Immun. 1985 Mar;47(3):827–830. doi: 10.1128/iai.47.3.827-830.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whoriskey S. K., Nghiem V. H., Leong P. M., Masson J. M., Miller J. H. Genetic rearrangements and gene amplification in Escherichia coli: DNA sequences at the junctures of amplified gene fusions. Genes Dev. 1987 May;1(3):227–237. doi: 10.1101/gad.1.3.227. [DOI] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Ludwig W. What are mycoplasmas: the relationship of tempo and mode in bacterial evolution. J Mol Evol. 1984;21(4):305–316. doi: 10.1007/BF02115648. [DOI] [PubMed] [Google Scholar]
- Yogev D., Rosengarten R., Watson-McKown R., Wise K. S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5' regulatory sequences. EMBO J. 1991 Dec;10(13):4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]