Abstract
Monocyte chemoattractant protein 1 (MCP-1) is a member of the chemokine cytokine family, whose physiological function is mediated by binding to the CCR2 and CCR4 receptors, which are members of the G protein-coupled receptor family. MCP-1 plays a critical role in both activation and migration of leukocytes. Rapid chemokine receptor desensitization is very likely essential for accurate chemotaxis. In this report, we show that MCP-1 binding to the CCR2 receptor in Mono Mac 1 cells promotes the rapid desensitization of MCP-1-induced calcium flux responses. This desensitization correlates with the Ser/Thr phosphorylation of the receptor and with the transient translocation of the G protein-coupled receptor kinase 2 (GRK2, also called β-adrenergic kinase 1 or βARK1) to the membrane. We also demonstrate that GRK2 and the uncoupling protein β-arrestin associate with the receptor, forming a macromolecular complex shortly after MCP-1 binding. Calcium flux responses to MCP-1 in HEK293 cells expressing the CCR2B receptor were also markedly reduced upon cotransfection with GRK2 or the homologous kinase GRK3. Nevertheless, expression of the GRK2 dominant-negative mutant βARK-K220R did not affect the initial calcium response, but favored receptor response to a subsequent challenge by agonists. The modulation of the CCR2B receptor by GRK2 suggests an important role for this kinase in the regulation of monocyte and lymphocyte response to chemokines.
Keywords: chemokines, arrestin
The chemokines are a large family of chemotactic cytokines that have a central role in leukocyte migration. The C-X-C chemokines act on neutrophils and nonhematopoietic cells involved in wound healing, whereas the C-C chemokines act on monocytes, eosinophils, basophils, natural killer (NK) cells, and diverse lymphocyte subpopulations. Chemokines interact with an array of G protein-coupled receptors (GPCRs) that can be grouped on the basis of their ability to bind to one or more C-X-C or C-C chemokines (1–4). The C-C chemokine monocyte chemoattractant protein 1 (MCP-1) was originally described as a potent chemoattractant for monocytes and is produced by several cell types in response to a variety of mediators (5, 6). Since then, many other activities have been assigned to MCP-1, including induction of T cell migration, tumor growth suppression in animal models, and HIV-1 neutralization (7–9).
Two MCP-1 receptor forms, the type A (CCR2A) and type B (CCR2B) CCR2 receptors, have recently been cloned and found to differ only in their carboxyl tails (10). In signaling studies, both mediate agonist-dependent calcium mobilization, as well as inhibition of adenylyl cyclase and cell migration through the coupling to pertussis toxin-sensitive G proteins (11). We have recently shown that Gαi coimmunoprecipitates with the CCR2 receptor in Mono Mac 1 cells (M.M., J.M.R.F., A.M.A., G. del Real, A.M.M., A. Serrano, F.M., and C.M.-A, unpublished work); however, it has been suggested that MCP-1 receptors, as well as those for other chemotactic chemokines such as RANTES (regulated on activation, normal T cell-expressed and secreted), may couple to multiple G proteins (12–14).
Little is known about the regulation mechanisms of the cellular response to chemokines, or of the role of desensitization in lymphocyte migration. The trafficking of different lymphocyte populations is a complex process controlled by a vast array of molecules. In this process, cells must be able to continuously sense small changes in chemoattractant gradients. Migration through a chemotactic gradient probably employs an on–off mechanism in which chemokine receptor desensitization may be an important step. For a large number of related GPCRs, rapid desensitization appears to involve agonist-promoted receptor phosphorylation by G protein-coupled receptor kinases (GRKs) (15–17). GRK-mediated phosphorylation of serine/threonine residues in the carboxyl tail and/or intracellular loops of GPCR increases the affinity for arrestin-type proteins, the binding of which prevents any further coupling between the receptor and G proteins (18, 19). The uncoupled receptors are subsequently removed from the plasma membrane in a process termed internalization. Recent evidence suggests that both GRK and arrestins also play a key role in the sequestration process (20, 21).
There are six known members of the GRK family, GRK1 to GRK6 (16–18). On the basis of sequence homology, they are classified into three groups: GRK1 (also known as rhodopsin kinase), GRK2 and -3 [also called β-adrenergic receptor kinase 1 and 2 (βARK1 and -2), respectively], and GRK-4, -5, and -6. Several unique features distinguish agonist-induced desensitization mediated by the second group. Cytosolic GRK2 and GRK3 are translocated to the membrane upon receptor activation in a process facilitated by interactions with released Gβγ dimers (22–24). Although GRK2, -3, -5, and -6 subtypes are ubiquitous, GRK2 is particularly abundant in peripheral blood leukocytes, as well as in myeloid and lymphoid cell lines (25). The physiological role of this high level of kinase expression has nevertheless not been explored in detail.
In this report, we provide evidence that the desensitization of the CCR2 receptor in monocytes is mediated by GRK2, which translocates to membranes upon MCP-1 stimulation and forms a macromolecular complex with the activated CCR2 receptor and the uncoupling protein β-arrestin. Moreover, the pattern of CCR2B receptor desensitization expressed in HEK293 cells can be modulated by the co-expression of GRK2, GRK3, or a dominant-negative GRK2 kinase mutant, suggesting an important role for these kinases in chemokine receptor desensitization.
MATERIALS AND METHODS
Biological Materials.
Mono Mac 1 cells (DSM ACC252) were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Human embryonic kidney (HEK-EBNA293) cells were obtained from Invitrogen. Antibodies for immunoprecipitation analysis include a mixture of anti-phosphoserine/threonine mAb (Biomol, Hamburg, Germany), anti-MHC class I mAb (W6/32, IgG2aκ, American Type Culture Collection,), anti-CD4 mAb (HP 2.6, IgG2bκ; kindly donated by A. Carrera, Centro Nacional Biotecnología, Madrid), anti-GRK2 antibody AbFP1 (21), anti-JAK2 polyclonal antibody (Upstate Biotechnology, Lake Placid, NY), and anti-CCR2 mAb MCP-1R03 (26). Antibodies for Western blot analysis include anti-GRK2 antibody AB9 (27) (a gift of J. L. Benovic, Jefferson University, Philadelphia), anti-GRK3 antibody (Santa Cruz Biotechnology), anti-arrestin mAb F4C1 (from L. Donoso, Wills Eye Hospital, Philadelphia), anti β-arrestin-1 antibody Ab186 (a generous gift of P. Penela, Centro de Biología Molecular, Madrid), and anti-CCR2 mAb MCP-1R05 (26). Recombinant human MCP-1 and stromal cell-derived factor 1α (SDF-1α) were obtained from PeproTech (London).
Cell Culture and Transfection.
Mono Mac 1 cells were maintained in RPMI medium 1640 containing 10% fetal bovine serum (FBS). HEK-EBNA293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS, and were transiently transfected by using Lipofectamine (GIBCO/BRL) and following the manufacturer’s instructions. cDNAs encoding human CCR2B (ref. 9; kindly donated by A. Zaballos, Centro Nacional Biotecnología, Madrid), CCR2B-IX (ref. 28; the generous gift of I. Charo, University of California, San Francisco), bovine GRK2 (29), βARK-K220R (30), human GRK5 and human GRK6 (the generous gifts of J.L. Benovic) cloned in pcDNA3, rat GRK3 cloned in pCMV (the generous gift of S. Cotecchia, Lausanne University), and control pcDNA3 were used for transfection. Cells (1.5 × 106 per 60-mm plate) were seeded the day before transfection and transfected with 5 μg of total plasmid DNA and 25 μl of Lipofectamine per plate. Empty pcDNA3 was used to equalize the total amount of cDNA used. Cells were collected 48 h after transfection in phosphate-buffered saline (PBS) containing 5 mM EDTA and resuspended in either DMEM or RPMI medium containing 10% FBS and 25 mM Hepes for calcium determinations or flow cytometry analysis. GRK kinase activity in cellular extracts of HEK293 cells cotransfected with the CCR2B receptor and GRK2, GRK3, or βARK-K220R was assayed by the rhodopsin phosphorylation assay as described before (27).
Calcium Determinations.
Changes in intracellular calcium concentration were monitored by using the fluorescent probe Fluo-3 (Calbiochem) as described (9, 26). Mono Mac 1 cells or transiently transfected HEK293 cells (2.5 × 106 cells per ml) were resuspended in RPMI or DMEM containing 10% FBS and 10 mM Hepes and were incubated with Fluo-3 (300 mM in dimethyl sulfoxide, 10 μl per 106 cells) for 15 min at 37°C. After incubation, cells were washed and resuspended in complete medium containing 2 mM CaCl2 and maintained at 37°C before addition of MCP-1 at various concentrations. Calcium release in response to MCP-1 at 5 nM or at the concentrations indicated in the figure legends (0.1, 1, 10, and 50 nM) was determined in an Epics XL flow cytometer at 525 nm. In some experiments, 10 nM MCP-1 was added a second time, as shown in the figures.
Immunoprecipitation, SDS/PAGE, and Western Blot Analysis.
For immunoprecipitation, Mono Mac 1 cells or transiently transfected HEK293 cells (20 × 106) were lysed in a detergent buffer [20 mM triethanolamine⋅hydrochloride, pH 8.0/300 mM NaCl/2 mM EDTA/20% (vol/vol) glycerol/1% digitonin, with protease inhibitors] for 30 min at 4°C with continuous rocking, followed by centrifugation (15,000 × g, 15 min). Immunoprecipitation was performed essentially as described in ref. 31. Protein extracts precleared by incubation with 20 μg of anti-mouse IgG-agarose (Sigma) or staphylococcal protein A-Sepharose (60 min, 4°C) were centrifuged (15,000 × g, 1 min) and incubated with the appropriate mAb or polyclonal antibody, respectively (5 μg per sample, 120 min, 4°C). Anti-mouse IgG-agarose was used to immunoprecipitate the anti-CCR2 (MCP-1R03) and anti-arrestin (F4C1) antibody complexes, whereas protein A-Sepharose was utilized for the anti-GRK2 antibody (AbFP1). Immunoprecipitates were resolved in SDS/12.5% PAGE and transferred to nitrocellulose membranes as described before (31). Single blots were first developed with anti-phosphoserine/phosphothreonine, anti-GRK2, or anti-arrestin antibodies, and then the same blot was reprobed with anti-CCR2 antibody as an internal control for the amount of protein loaded in each gel. For Western blot analysis of HEK293 transiently transfected cells or Mono Mac cell lysates, cells were washed with PBS and lysed by Dounce homogenization, and protein concentration was determined by the Lowry method. Gels were loaded with 5 μg of cell lysate proteins from transiently transfected HEK293 cells or with 40 μg of protein from Mono Mac 1 cell lysates or Mono Mac 1 subcellular fractions. In some experiments, soluble and particulate Mono Mac 1 cell fractions were obtained under different experimental conditions, and the subcellular GRK2 distribution was assessed in Western blots as reported (32). After fractionation the protein concentration in each sample was determined by the Lowry method and equal amounts of protein were loaded onto the gels. Usually, only the relevant region of the gel is shown in the immunoblots.
RESULTS AND DISCUSSION
MCP-1 Triggers Association of GRK2, Arrestin, and the CCR2 Receptor in a Multimolecular Complex.
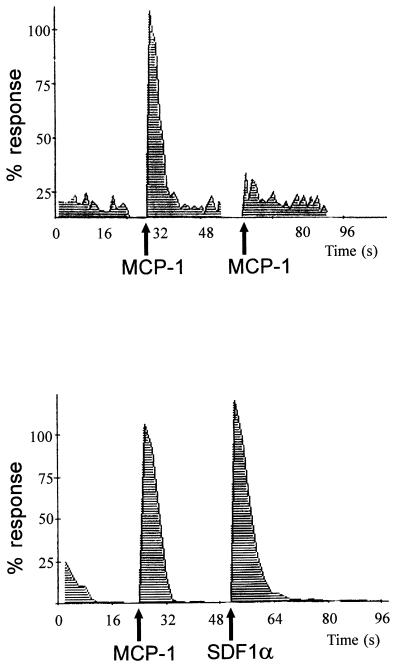
We have previously demonstrated that Mono Mac 1 cells express the MCP-1 chemokine receptor CCR2 (9, 26). These cells respond to MCP-1 by inducing calcium mobilization (Fig. 1) and cell migration (not shown). The calcium response to a second challenge of 10 nM MCP-1 is markedly reduced, suggesting rapid CCR2 receptor desensitization (Fig. 1 Upper). The response to another chemokine (SDF-1α) acting through a different receptor (CXCR4) was not affected by the first MCP-1 challenge (Fig. 1 Lower), thus indicating homologous CCR2 receptor desensitization.
Figure 1.
MCP-1-induced desensitization of the CCR2B receptor. Calcium influx was promoted by the addition of 10 nM human MCP-1 to Mono Mac 1 cells loaded with Fluo-3; a second challenge with 10 nM MCP-1 promoted very little calcium response (Upper). As a control, MCP-1-induced cells were stimulated with 10 nM SDF-1α (Lower). Calcium influx in response to MCP-1 was determined by flow cytometry at 525 nm, as detailed in the text. Data are given as a percentage of maximum MCP-1 fluorescence response. Arrows depict the time of chemokine addition.
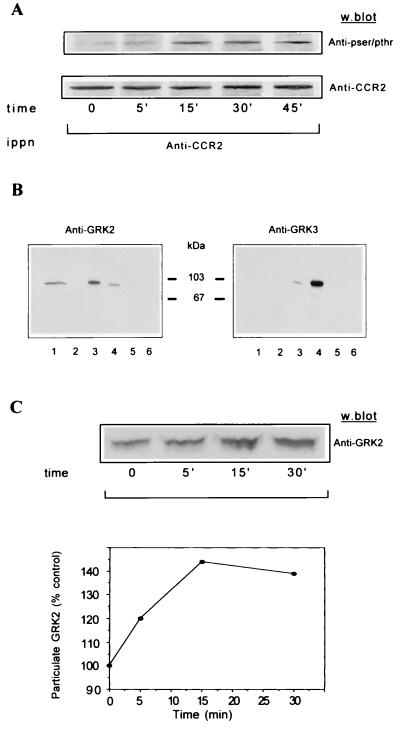
The rapid uncoupling of other GPCRs is mediated by agonist-promoted phosphorylation by GRKs (15–18). Analysis of the CCR2 receptor carboxyl-terminal sequence reveals serine and threonine residues that could be potential sites for phosphorylation by these receptor kinases. We therefore asked whether treatment of Mono Mac 1 cells with MCP-1 promotes CCR2 receptor phosphorylation in Ser/Thr residues. Cells treated for different times with MCP-1 were lysed, and extracts were immunoprecipitated with the CCR2 receptor-specific antibody (MCP-1R03). Western blot analysis of immunoprecipitates with a mixture of anti-phosphoserine/threonine mAbs showed a phosphorylated protein in the position (38 kDa) corresponding to that of the CCR2 receptor (Fig. 2A Upper). The analysis shows trace receptor phosphorylation under basal conditions, and a rapid increase as early as 5 min after MCP-1 stimulation, reaching maximal phosphorylation at 15–30 min and decreasing thereafter. Equal protein loading was confirmed by developing the same Western blot with the anti-CCR2 MCP-1R05 mAb (Fig. 2A Lower).
Figure 2.
(A) Time course of serine/threonine phosphorylation of the CCR2B receptor in Mono Mac 1 cells. Mono Mac 1 cells were stimulated for the times indicated and lysed, and cell extracts were immunoprecipitated with an anti-CCR2 antibody. After SDS/PAGE and transfer, the same blot was first developed with a mixture of anti-phosphoserine/threonine antibodies (Upper) and then reprobed with the anti-CCR2 antibody MCP-1R05 (Lower). The molecular mass of the CCR2 receptor is 38 kDa. The figure is representative of three experiments with similar results. (B) Western blot analysis of the presence of GRK2 and GRK3 in Mono Mac 1 cell extracts (lane 1). For comparative purposes, the same analysis was performed in HEK293 cells transiently transfected with pcDNA3, GRK2, GRK3, GRK5, or GRK6 (lanes 2–6, respectively). Blots were tested with the anti-GRK2 AB9 (Left) and anti-GRK3 antibodies (Right). The molecular mass of GRK2 and GRK3 is ≈80 kDa and 78 kDa, respectively. (C) Western blot analysis of MCP-1-induced GRK2 translocation in Mono Mac 1 cells. Cells were stimulated with MCP-1 for the times indicated and particulate fractions were obtained as described in the text. Equal protein amounts were resolved in SDS/PAGE, blotted, and developed with anti-GRK2 AB9 antibody (Upper). Data were quantitated by laser densitometry, normalized by the signal of an unrelated band, and represented as the percentage of particulate GRK2 before stimulation (Lower). This experiment was repeated twice with similar results.
Given the reported high GRK2 expression levels in peripheral blood leukocytes and in myeloid and lymphoid cell lines (25), we analyzed whether GRK2 (βARK1) was present in Mono Mac 1 cells and its potential participation in the observed Ser/Thr phosphorylation of the receptor. Western blot analysis of Mono Mac 1 cell lysates (Fig. 2B Left, lane 1) and lysates from 293 cells transfected with different GRKs (lanes 2–6) with an antibody raised against GRK2 indicates marked expression of this kinase in the Mono Mac cell line. The GRK2 AB9 antibody shows slight cross-reactivity with GRK3, but this band migrates at a slightly different molecular mass. Analysis with an antibody directed against GRK3, which also shows a slight cross-reactivity with GRK2 (Fig. 2B Right, lanes 3–4), did not show any significant expression of GRK3 in Mono Mac 1 cells. Since GRK2 translocates to the periphery of the membrane after ligand activation of GPCR to phosphorylate the receptor (25, 33), we analyzed GRK2 distribution in particulate fractions before and after MCP stimulation (Fig. 2C). Addition of MCP-1 promotes a transient increase in the amount of GRK2 in the particulate fraction, which reaches a maximum at approximately 15 min after MCP-1 stimulation. These data are consistent with the time course of CCR2 receptor phosphorylation (Fig. 2A) and are similar to those found for other receptors (25, 32, 33). The modest changes in subcellular GRK2 distribution may reflect the fact that only a small fraction of cytoplasmic GRK2 associates with the ligand-stimulated receptor in the plasma membrane, as well as the rapid intracellular dynamics of this kinase (21). It should also be noted that a significant fraction of GRK2 is associated with the particulate fraction under nonstimulation conditions. This observation could be ascribed both to its reported association with microsomal membranes (27, 32, 34) and to its binding to the plasma membrane as a consequence of the basal GPCR activity (21, 35), which may contribute to rapid receptor regulation after agonist binding.
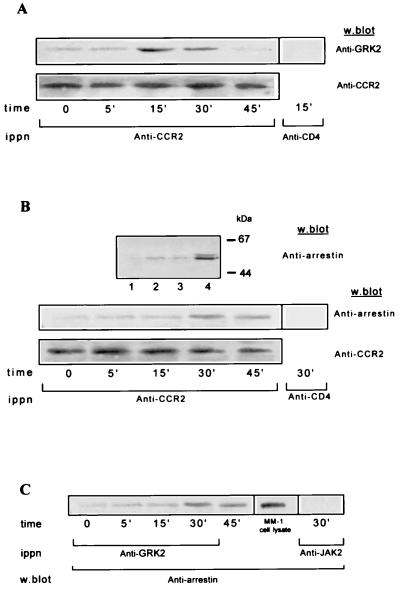
To further show a functional relationship between GRK2 and this chemokine receptor, we next asked whether GRK2 could be found associated with the CCR2 receptor in Mono Mac 1 cells. We immunoprecipitated extracts from MCP-1-stimulated cells with an anti-CCR2 receptor antibody and tested for the presence of GRK2 in Western blots. GRK2 associates with the CCR2 receptor in an MCP-1-dependent manner; this association is most evident at 15–30 min after MCP-1 induction (Fig. 3A Upper). Assay specificity was demonstrated by immunoprecipitating the same cell extracts with an unrelated mAb used as control (anti-CD4). The changes in GRK2 association are not the consequence of variations in the CCR2 receptor immunoprecipitation, since the Western blot analysis of the same blot with the anti-CCR2 antibody MCP-1R05 confirms equal CCR2 protein loading (Fig. 3A Lower). Immunoprecipitation with an anti-GRK2 antibody (AbFP1) followed by an anti-CCR2 receptor antibody (MCP-1R05) immunoblot analysis gave comparable results (not shown). These data demonstrate that GRK2 forms a macromolecular complex with the CCR2 receptor after MCP-1 activation.
Figure 3.
Association of GRK2 and β-arrestin to the MCP-1-stimulated CCR2B receptor in Mono Mac 1 cells. (A and B) Whole cell lysates from Mono Mac 1 cells stimulated with MCP-1 for the times indicated were precipitated with anti-CCR2 MCP-1R03 antibody. Immunoprecipitates were first analyzed in a Western blot with anti-GRK2 AB9 (A Top) or with anti-arrestin (B Middle) antibodies, and the same blots were then developed with anti-CCR2 MCP-1R05 (A and B Lower) antibodies, as indicated. In B Upper, lysates from mock or β-arrestin1-transfected HEK293 cells (lanes 1 and 2, respectively) or Mono Mac 1 particulate and cytosolic fractions (lanes 3 and 4, respectively), were analyzed in a Western blot with an anti-arrestin mAb. The molecular mass of the β-arrestin1 is ≈50 kDa. (C) MCP-1-stimulated Mono Mac 1 cell lysates were immunoprecipitated with anti-GRK2 antibody and analyzed by Western blotting with anti-arrestin antibody. A Mono Mac 1 cell lysate (MM-1) was included as a control of β-arrestin migration in the same gel. As negative controls, MCP-1-stimulated cell lysates were immunoprecipitated with an anti-CD4 mAb (A and B) or anti-JAK2 polyclonal antibodies (C), and Western blots were stained with the indicated antibody. Results are representative of three experiments with similar results.
We next tested the presence of arrestin-like proteins in Mono Mac 1 cells. For this, β-arrestin1-transfected HEK293 and Mono Mac 1 cell lysates were analyzed by Western blotting with an anti-arrestin mAb (Fig. 3B Top) or a specific β-arrestin1 polyclonal antibody (not shown). The results demonstrate the presence of a predominantly cytosolic protein of ≈50 kDa corresponding to β-arrestin1 in Mono Mac 1 cells (Fig. 3B, lanes 3 and 4). We therefore studied MCP-1-induced association of β-arrestin to CCR2 receptors. Mono Mac 1 cell extracts were immunoprecipitated with an anti-CCR2 receptor antibody and analyzed in a Western blot with the monoclonal anti-arrestin antibody (Fig. 3B Middle). A protein recognized by the anti-arrestin antibody coimmunoprecipitates with the MCP-1-stimulated receptor; this association reaches a maximum 30 min after MCP-1 stimulation and slightly decreases by 45 min. Equal protein loading in the gel was assessed by reprobing the Western blot with an anti-CCR2 antibody (Fig. 3B Bottom). Control experiments with an unrelated antibody (anti-CD4) confirm the specificity of the β-arrestin–CCR2 receptor interaction. The time course of arrestin association with the chemokine receptor is retarded with regard to that of GRK2, consistent with the requirement for GRK-mediated receptor phosphorylation for β-arrestin1 binding to GPCR.
Because both GRK2 and arrestin proteins can be found associated with the CCR2 receptor, it is possible that β-arrestin forms part of a macromolecular complex with the receptor and the kinase. We thus performed immunoprecipitation studies with an anti-GRK2 antibody (AbFP1), followed by Western blot analysis with an anti-arrestin mAb. β-arrestin coimmunoprecipitates with GRK2 in an MCP-1-dependent manner (Fig. 3C), strongly suggesting that both the kinase and the uncoupling protein are present simultaneously in the same, presumably multimolecular, receptor complex.
GRK2 Controls MCP-1-Mediated CCR2B Receptor Signaling.
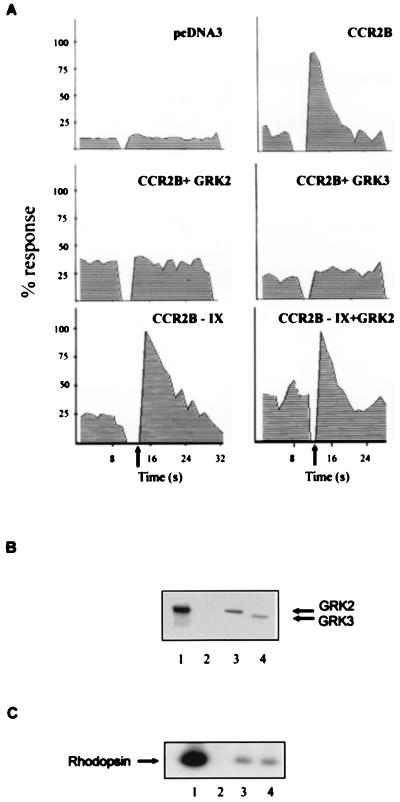
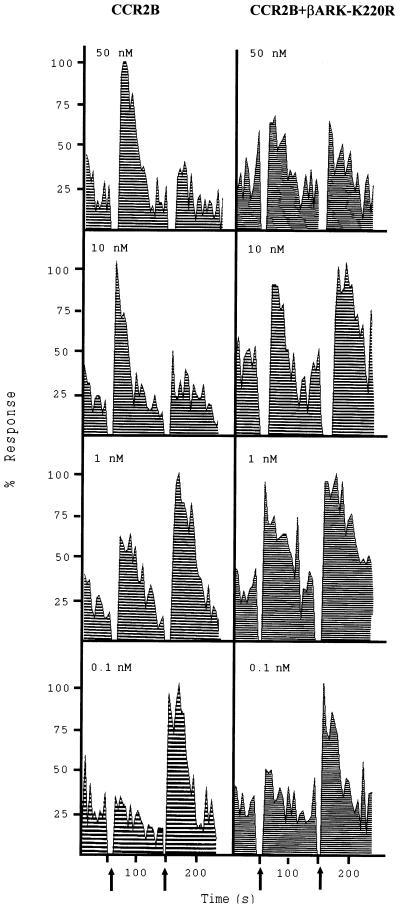
To further establish the role of GRK2 in CCR2 receptor desensitization, we analyzed the effect of chemokine receptor coexpression with GRK2 in transfection experiments. CCR2B receptor expression was assessed in HEK293 cells by flow cytometry and Western blot analysis using mAb specific for the CCR2B receptor, as described (26) (not shown). MCP-1 stimulation of CCR2B-transfected HEK293 cells produced a transient calcium increase (Fig. 4A and Fig. 5). When cells were cotransfected with GRK2 the response to MCP-1 was completely inhibited. Similar results were obtained when the homologous GRK3 was co-expressed with the receptor. The expression and activity of both kinases in transiently cotransfected HEK293 cells was confirmed by Western blot analysis (Fig. 4B) and by the rhodopsin phosphorylation assay (Fig. 4C). Both kinases showed similar levels of rhodopsin phosphorylation. Taken together, these results suggest that both GRK2 and GRK3 are able to modulate CCR2B receptor signaling with comparable effectivities. Interestingly, when a CCR2B receptor mutant that lacks all Ser/Thr residues in the carboxyl tail (CCR2B-IX, see ref. 28) was expressed, the MCP-1-induced signal was not inhibited by GRK2 co-expression (Fig. 4A). GRK2 levels were similar in CCR2B and CCR2B-IX receptor-expressing cells (data not shown). Additional support for the hypothesis that GRK2 is critically involved in CCR2B receptor deactivation is provided by experiments (Fig. 5) in which the CCR2B receptor was co-expressed with a dominant-negative mutant of GRK2, βARK-K220R (30). Expression of this mutant in HEK293 cells at levels similar to those attained with wild-type GRK2 does not lead to a significant increase in kinase activity as assessed by the rhodopsin assay (data not shown). In the presence of GRK2-K220R, MCP-1 induced at different concentrations a calcium response comparable to that observed with the receptor alone (Fig. 5), clearly different from the signal block observed when wild-type GRK2 is co-expressed (Fig. 4A). The calcium transient observed in GRK2-K220R-transfected cells was sometimes partially reduced in response to MCP-1, probably reflecting the interaction of the mutant kinase with cellular proteins downstream of the receptor (see below). More important, cells expressing GRK2-K220R exhibited a response to a second challenge with 10 nM MCP-1 that was equivalent to the original response even when higher concentrations of ligand were tested, conditions at which the response of cells transfected with the receptor alone is desensitized (Fig. 5). Such similar response to a second challenge of ligand was previously observed by others (36) when expressing a carboxyl-terminal truncated form of a CXCR2 receptor or a receptor mutated on serine and threonine residues, using the same experimental design.
Figure 4.
GRK2 and GRK3 modulation of MCP-1 signaling mediated by the CCR2B receptor. (A) HEK293 cells were transiently transfected with pcDNA3 alone; with pcDNACCR2B with pcDNA3, pcDNAGRK2, or pCMVGRK3; or with pcDNACCR2B-IX and pcDNA3 or pcDNAGRK2 as indicated. Calcium was determined as described in the legend of Fig. 1. Data are given as a percentage of maximal MCP-1-induced response. Arrows indicate the time of MCP-1 addition. (B) Western blot analysis of the expression level of GRK2 and GRK3 in transiently cotransfected HEK293 cells. Recombinant GRK2 (lane 1) and lysates from CCR2B, CCR2B + GRK2, and CCR2B + GRK3 cells (lanes 2–4, respectively) were resolved by SDS/PAGE and transferred, and blots were developed simultaneously with anti-GRK2 AB9 and anti-GRK3 antibodies. (C) HEK293 cells cotransfected with the CCR2B receptor alone (lane 2) or with either GRK2 (lane 3) or GRK3 (lane 4) were lysed, and the cytosolic kinase activity was determined with rhodopsin as substrate. Recombinant GRK2 (10 nM) was used as a control (lane 1). A representative autoradiogram is shown. Results in all panels are representative of two independent experiments.
Figure 5.
βARK-K220R inhibition of MCP-1-induced desensitization of the CCR2B receptor. HEK293 cells were transiently cotransfected with pcDNACCR2B and either empty vector or pcDNAβARK-K220R. Cells were stimulated first with different concentrations of MCP-1 as indicated. After approximately 2 min, 10 nM MCP-1 was added a second time as indicated by the arrows. Calcium was determined as described in the legend of Fig. 1. Data are given as a percentage of maximal MCP-1-induced and are representative of two independent experiments.
The effects of GRK2 overexpression on CCR2B receptor-induced calcium signaling can be explained in two ways. First, increasing GRK2 or GRK3 concentration in cells would favor its association to receptors, thus dampening its response to ligand by increasing receptor phosphorylation or by directly preventing interaction with G proteins (37). We tested whether GRK2 association with the CCR2B receptor could be observed in HEK293 cotransfected cells. When cell lysates from unstimulated HEK293 cells were immunoprecipitated with anti-CCR2 receptor antibodies and analyzed with an anti-GRK2 antibody, the kinase coimmunoprecipitated with the receptor, demonstrating that overexpressed GRK2 associates with the receptor, even in the absence of ligand (not shown). The receptor is also bound to the nonfunctional kinase in βARK-K220R-transfected HEK293 cells (not shown). In this case, because the GRK2 mutant lacks kinase activity but retains its receptor binding capacity, its interaction with the CCR2B receptor would inhibit any further association and phosphorylation by endogenous kinases, preventing receptor desensitization. Because the carboxyl-terminal domain of GRK2 and -3 can interact with both G protein βγ subunits and phosphatidylinositol 4,5-bisphosphate (24, 38), an additional effect of kinase overexpression could be the blockage of Gβγ-mediated activation of phospholipase C β (37, 39). This effect would also apply to the βARK-K220R dominant-negative mutant, which maintains this carboxyl-terminal domain intact. Alternatively, kinase binding would compete with the association of the G protein heterotrimer with the receptor. Consistently, MCP-1-induced calcium response in cells coexpressing the receptor and βARK-K220R was detected only when the total amount of the mutant kinase was maintained below 0.3–0.4 μg of cDNA per 105 cells transfected. On the other hand, “low” expression of GRK2 with the CCR2B-IX mutated receptor, which lacks all Ser/Thr residues susceptible to phosphorylation by GRK2 (28), did not inhibit the calcium response (see Fig. 4A), thus demonstrating that the GRK2 inhibitory effect is truly mediated by receptor phosphorylation. Comparable results were obtained by Diviani et al. (39) when investigating epinephrine-induced phosphatidylinositol hydrolysis. In summary, the data presented here suggest that GRK2 and GRK3 modulate the CCR2B receptor signaling by direct interaction with the receptor. The lower expression levels of GRK3 in Mono Mac 1 cells compared with GRK2 suggest a key role for the latter in modulating the chemokine receptor response in monocytes.
Concluding Remarks.
Desensitization and recycling of chemotactic receptors appears to be a critical mechanism by which leukocytes maintain their ability to sense the chemoattractant gradient in the inflammatory response. Previous studies demonstrated that chemotactic receptors of the C-X-C family were also phosphorylated on Ser residues by GRK enzymes (40–42). In this report, we show that the chemotactic receptor CCR2, a member of the C-C family, is regulated in intact cells by GRK2. It has recently been shown that the CCR2B receptor tail is responsible for receptor desensitization in the Xenopus oocyte expression system (28), in which GRK-3 appears to be the kinase responsible for receptor phosphorylation. In transfected HEK293 cells we show that both GRK2 and GRK3 are capable of modulation this chemokine receptor. Nevertheless, GRK3 appears to be expressed at very low levels in Mono Mac 1 cells, strongly indicating that GRK2 would be the physiologically relevant kinase in this cell type.
Interestingly, the CCR2 chemokine receptor, GRK2, and the regulatory protein β-arrestin are found associated after MCP-1 stimulation. Recent reports have shown that both GRK2 (21) and arrestin (35) colocalize with β-adrenergic receptors in internalization vesicles. Although our experimental approach does not permit localization of the receptor macromolecular complex in the plasma membrane or in endocytic vesicles, our data support the hypothesis that both GRK2 and arrestin associate with the receptor shortly after agonist stimulation and during the sequestration process. Such a mechanism may also be used for other GPCR, GRK, and arrestin proteins.
The ability of GRK2 to modulate a C-C-chemokine receptor is consistent with the high expression levels of this kinase in monocytes, granulocytes, and lymphoid cell lines (25), and with a key role for desensitization processes in the modulation of lymphocyte migration. Our data suggest that GRK enzymes play an essential physiological role in the modulation of cell signaling through diverse chemokine receptors. Modulation of GRK2 activity and expression thus emerges as an additional means to regulate cell responses to chemokines.
Acknowledgments
We thank J. L. Benovic, L. Donoso, I. Charo, A. Serrano, A. Zaballos, A. Carrera, A. Ruiz-Gómez, and A. Elorza for suggestions and experimental tools, M. C. Moreno and I. López for help with calcium determinations, E. Montoya and M. J. Toro for critical reading of the manuscript, and M. Sanz and C. Mark for editorial assistance. This work was partially supported by grants from the Dirección General de Investigación Científica y Técnica/PM-95–0033, Boehringer Ingelheim, and the European Union to F.M., and by grants from the Dirección General de Investigación Científica y Técnica and the European Union to C.M.-A. The Departamento de Inmunología y Oncología was founded and is supported by the Consejo Superior de Investigaciones Científicas, and by Pharmacia and Upjohn. The Centro de Biología Molecular receives an institutional grant from the Fundación Ramón Areces.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: GRK, G protein-coupled receptor kinase; GPCR, G protein-coupled receptor; Gβγ, βγ subunits of heterotrimeric G proteins; βARK, β-adrenergic receptor kinase; MCP-1, monocyte chemoattractant protein 1; SDF-1α, stromal cell-derived factor 1α; HEK293, human embryonic kidney cells.
References
- 1.Murphy P M. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 2.Schall T J. In: The Cytokine Handbook. Thompson A W, editor. London: Academic; 1994. pp. 419–460. [Google Scholar]
- 3.Howard O M Z, Ben-Baruch A, Oppenheim J J. Trends Biotechnol. 1996;14:46–51. doi: 10.1016/0167-7799(96)80920-6. [DOI] [PubMed] [Google Scholar]
- 4.Hedrick J A, Zlotnik A. Curr Opin Immunol. 1996;8:343–347. doi: 10.1016/s0952-7915(96)80123-3. [DOI] [PubMed] [Google Scholar]
- 5.Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sossanis S, Coletta I, Mantovani A. J Immunol Methods. 1994;174:249–257. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini M, Dewald B, Moser B. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 7.Flory C M, Jones M L, Warren J S. Lab Invest. 1993;69:396–404. [PubMed] [Google Scholar]
- 8.Carr M W, Roth S J, Luther E, Rose S S, Springer T A. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frade J M R, Llorente M, Mellado M, Alcami J, Gutierrez-Ramos J C, Zaballos A, Real G del, Martinez-A C. J Clin Invest. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charo I F, Myers S J, Herman A, Franci C, Connolly A J, Coughlin S R. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong L-M, Myers S J, Tsou C-L, Gosling J, Arai H, Charo I F. J Biol Chem. 1997;272:1038–1045. doi: 10.1074/jbc.272.2.1038. [DOI] [PubMed] [Google Scholar]
- 12.Arai H, Charo I F. J Biol Chem. 1996;271:21814–21819. doi: 10.1074/jbc.271.36.21814. [DOI] [PubMed] [Google Scholar]
- 13.Kuang Y, Wu Y, Jiang H, Wu D. J Biol Chem. 1996;271:3975–3978. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- 14.Al-Aoukaty A, Schall T J, Maghazachi A A. Blood. 1996;87:4255–4260. [PubMed] [Google Scholar]
- 15.Lefkowitz R J. Cell. 1993;74:409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- 16.Premont R T, Inglese J, Lefkowitz R J. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 17.Lohse M J, Krasel C K, Winstel R, Mayor F., Jr Kidney Int. 1996;49:1047–1052. doi: 10.1038/ki.1996.153. [DOI] [PubMed] [Google Scholar]
- 18.Bohm S K, Grady E F, Bunnett N W. Biochem J. 1997;322:1–8. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne-Marr R, Benovic J L. Vitam Horm. 1995;51:193–234. doi: 10.1016/s0083-6729(08)61039-0. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson S S G, Downey W E, III, Colapietro A M, Barak L S, Menard L, Caron M. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Gómez A, Mayor F., Jr J Biol Chem. 1997;272:9601–9604. doi: 10.1074/jbc.272.15.9601. [DOI] [PubMed] [Google Scholar]
- 22.Pitcher J A, Inglese J, Higgins J B, Arriza J L, Casey P J, Kim C, Benovic J L, Kwatra M M, Caron M G, Lefkowitz R J. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 23.Kameyama K, Haga K, Haga T, Kotani T, Fukada Y. J Biol Chem. 1993;268:7753–7758. [PubMed] [Google Scholar]
- 24.Koch W J, Inglese J, Stone W C, Lefkowitz R J. J Biol Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 25.Chuang T T, Sallese M, Ambrosini G, Parruti G, De Blasi A. J Biol Chem. 1992;267:6886–6892. [PubMed] [Google Scholar]
- 26.Rodríguez-Frade J M, Mellado M, Real G del, Gutiérrez-Ramos J C, Lind P, Martínez-A C. J Immunol. 1997;159:5576–5584. [PubMed] [Google Scholar]
- 27.García-Higuera I, Penela P, Murga C, Egea G, Bonay P, Benovic J L, Mayor F., Jr J Biol Chem. 1994;269:1348–1355. [PubMed] [Google Scholar]
- 28.Franci C, Gosling J, Tsou C-L, Coughlin S R, Charo I F. J Immunol. 1996;157:5606–5612. [PubMed] [Google Scholar]
- 29.Benovic J L, De Blasi A, Stone W, C, Caron M G, Lefkowitz R J. Science. 1989;246:235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- 30.Kong G, Penn R, Benovic J L. J Biol Chem. 1994;269:13084–13087. [PubMed] [Google Scholar]
- 31.Mellado M, Rodríguez-Frade J M, Kremer L, Von Kobbe C, Martin de Ana A, Merida I, Martinez-A C. J Biol Chem. 1997;272:9189–9196. doi: 10.1074/jbc.272.14.9189. [DOI] [PubMed] [Google Scholar]
- 32.García-Higuera I, Mayor F., Jr J Clin Invest. 1994;93:937–943. doi: 10.1172/JCI117099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayor F, Jr, Benovic J L, Caron M C, Lefkowitz R J. J Biol Chem. 1987;262:6468–6471. [PubMed] [Google Scholar]
- 34.Murga, C., Ruiz-Gómez, A., García-Higuera, I., Kim, C. M., Benovic, J. L. & Mayor, F., Jr. (1996) 271, 985–994. [DOI] [PubMed]
- 35.Goodman O B, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon W W, Kenn J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 36.Mueller S G, White J R, Schraw W P, Lam V, Richmond A. J Biol Chem. 1997;272:8207–8214. doi: 10.1074/jbc.272.13.8207. [DOI] [PubMed] [Google Scholar]
- 37.Oppermann M, Freedman N J, Alexander R W, Lefkowitz R J. J Biol Chem. 1996;271:13266–13272. doi: 10.1074/jbc.271.22.13266. [DOI] [PubMed] [Google Scholar]
- 38.DebBurman S K, Ptasienski J, Benovic J L, Hosey M M. J Biol Chem. 1996;271:22552–22562. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- 39.Diviani D, Lattion A-L, Larbi N, Kunapuli P, Pronin A, Benovic J L, Cotecchia S. J Biol Chem. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 40.Tardif M, Mery L, Brouchon L, Boulay F. J Immunol. 1993;150:3534–3545. [PubMed] [Google Scholar]
- 41.Ali H, Richardson R M, Tomhave E D, Didsbury J R, Snyderman R. J Biol Chem. 1993;268:24247–24252. [PubMed] [Google Scholar]
- 42.Prossnitz E R, Kim C M, Benovic J L, Ye R D. J Biol Chem. 1995;270:1130–1137. doi: 10.1074/jbc.270.3.1130. [DOI] [PubMed] [Google Scholar]