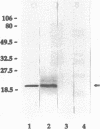
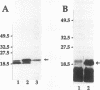
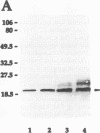
Abstract
Antisera raised in rabbits to whole cells of Helicobacter pylori recognized as a major antigen a protein with an apparent molecular weight of 20,000. The antigen was purified by differential solubilization with N-octyl-beta-D-glucopyranoside, urea, and sodium dodecyl sulfate followed by molecular sieving. The mass of the protein, Lpp20, was 18,283 Da as determined by mass spectrometry. The lpp20 gene encoding this protein was cloned in Escherichia coli by using the vector lambda EMBL3, and plasmid subclones expressed the full-length protein from the native H. pylori promoter. lpp20 was mapped to the same 358-kb NruI fragment as flaB. DNA sequence analysis showed that the gene was 525 bp long and encoded a 175-amino-acid protein with a molecular weight of 19,094 containing a 21-residue typical lipoprotein signal peptide and consensus prolipoprotein processing site. The mass of the deduced 154-residue mature protein was 16,865 Da. Growth of E. coli cells expressing the cloned H. pylori lpp20 gene in the presence of [3H]palmitic acid resulted in radiolabelled Lpp20 while treatment of the E. coli cells with globomycin caused accumulation of unprocessed Lpp20, consistent with Lpp20 being a lipoprotein. Lpp20 cofractionated with the cytoplasmic membrane fraction, although a proportion of the protein was also found in the outer membrane. A mutant generated by mutant-allele exchange displayed normal viability, showing that Lpp20 belonged to the nonessential class of lipoproteins.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. E., Baumstark B. R., Bellini W. J. Expression of the gene encoding the 17-kilodalton antigen from Rickettsia rickettsii: transcription and posttranslational modification. J Bacteriol. 1988 Oct;170(10):4493–4500. doi: 10.1128/jb.170.10.4493-4500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Rao J. K., Hargrave P. A. Structural prediction of membrane-bound proteins. Eur J Biochem. 1982 Nov 15;128(2-3):565–575. doi: 10.1111/j.1432-1033.1982.tb07002.x. [DOI] [PubMed] [Google Scholar]
- Austin J. W., Doig P., Stewart M., Trust T. J. Structural comparison of urease and a GroEL analog from Helicobacter pylori. J Bacteriol. 1992 Nov;174(22):7470–7473. doi: 10.1128/jb.174.22.7470-7473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J. The nicotinic cholinergic receptor : different compositions evidenced by statistical analysis. Biochem Biophys Res Commun. 1975 Jan 20;62(2):407–414. doi: 10.1016/s0006-291x(75)80153-7. [DOI] [PubMed] [Google Scholar]
- Bessler W. G., Jung G. Synthetic lipopeptides as novel adjuvants. Res Immunol. 1992 Jun;143(5):548–580. doi: 10.1016/0923-2494(92)80067-u. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori: microbiology of a 'slow' bacterial infection. Trends Microbiol. 1993 Oct;1(7):255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992 Feb;102(2):720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- Béji A., Mégraud F., Vincent P., Gavini F., Izard D., Leclerc H. GC content of DNA of Campylobacter pylori and other species belonging or related to the genus Campylobacter. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):527–534. doi: 10.1016/0769-2609(88)90152-4. [DOI] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanyangam M., Smith A. L., Moseley S. L., Kuehn M., Jenny P. Contribution of a 28-kilodalton membrane protein to the virulence of Haemophilus influenzae. Infect Immun. 1991 Feb;59(2):600–608. doi: 10.1128/iai.59.2.600-608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Henning U. Nucleotide sequence of the gene for the peptidoglycan-associated lipoprotein of Escherichia coli K12. Eur J Biochem. 1987 Feb 16;163(1):73–77. doi: 10.1111/j.1432-1033.1987.tb10738.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J., Ray P. H. Inhibition of prolipoprotein signal peptidase by globomycin. J Biol Chem. 1985 May 25;260(10):5891–5894. [PubMed] [Google Scholar]
- Doig P., Trust T. J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect Immun. 1994 Oct;62(10):4526–4533. doi: 10.1128/iai.62.10.4526-4533.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet E. B., Denoyel G. A., Boude M., Wallano E., Andujar M., de Montclos H. P. Characterization of an immunoreactive species-specific 19-kilodalton outer membrane protein from Helicobacter pylori by using a monoclonal antibody. J Clin Microbiol. 1991 Aug;29(8):1620–1624. doi: 10.1128/jcm.29.8.1620-1624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Dunn B. E., Perez-Perez G. I., Blaser M. J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989 Jun;57(6):1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Roop R. M., 2nd, Sung C. C., Sharma S. A., Perez-Perez G. I., Blaser M. J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992 May;60(5):1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Evans D. G., Karjalainen T. K., Evans D. J., Jr, Graham D. Y., Lee C. H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993 Feb;175(3):674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier B. A., Pfeifer J. D., Russell D. G., Falk P., Olsén A. N., Hammar M., Westblom T. U., Normark S. J. Paracrystalline inclusions of a novel ferritin containing nonheme iron, produced by the human gastric pathogen Helicobacter pylori: evidence for a third class of ferritins. J Bacteriol. 1993 Feb;175(4):966–972. doi: 10.1128/jb.175.4.966-972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gennity J. M., Kim H., Inouye M. Structural determinants in addition to the amino-terminal sorting sequence influence membrane localization of Escherichia coli lipoproteins. J Bacteriol. 1992 Apr;174(7):2095–2101. doi: 10.1128/jb.174.7.2095-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giam C. Z., Chai T., Hayashi S., Wu H. C. Prolipoprotein modification and processing in Escherichia coli. A unique secondary structure in prolipoprotein signal sequence for the recognition by glyceryl transferase. Eur J Biochem. 1984 Jun 1;141(2):331–337. doi: 10.1111/j.1432-1033.1984.tb08196.x. [DOI] [PubMed] [Google Scholar]
- Green B. A., Quinn-Dey T., Zlotnick G. W. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun. 1987 Dec;55(12):2878–2883. doi: 10.1128/iai.55.12.2878-2883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Holley L. H., Karplus M. Protein secondary structure prediction with a neural network. Proc Natl Acad Sci U S A. 1989 Jan;86(1):152–156. doi: 10.1073/pnas.86.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Duffaud G., Inouye M. Structural requirement at the cleavage site for efficient processing of the lipoprotein secretory precursor of Escherichia coli. J Biol Chem. 1986 Aug 25;261(24):10970–10975. [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kostrzynska M., Betts J. D., Austin J. W., Trust T. J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991 Feb;173(3):937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowka S., Eaton K. A., Rings D. M., Morgan D. R. Gastritis induced by Helicobacter pylori in gnotobiotic piglets. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 8):S681–S685. doi: 10.1093/clinids/13.supplement_8.s681. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Weis J. J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993 Sep;61(9):3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoa F. J., Miller K. W., Beers R., Graham M., Wu H. C. Membrane topology of Escherichia coli prolipoprotein signal peptidase (signal peptidase II). J Biol Chem. 1991 Sep 15;266(26):17667–17672. [PubMed] [Google Scholar]
- Nair J., Rouse D. A., Morris S. L. Nucleotide sequence analysis and serologic characterization of a 27-kilodalton Mycobacterium intracellulare lipoprotein. Infect Immun. 1993 Mar;61(3):1074–1081. doi: 10.1128/iai.61.3.1074-1081.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedenskov-Sørensen P., Bukholm G., Bøvre K. Natural competence for genetic transformation in Campylobacter pylori. J Infect Dis. 1990 Feb;161(2):365–366. doi: 10.1093/infdis/161.2.365. [DOI] [PubMed] [Google Scholar]
- Nelson M. B., Apicella M. A., Murphy T. F., Vankeulen H., Spotila L. D., Rekosh D. Cloning and sequencing of Haemophilus influenzae outer membrane protein P6. Infect Immun. 1988 Jan;56(1):128–134. doi: 10.1128/iai.56.1.128-134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole P. W., Austin J. W., Trust T. J. Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol Microbiol. 1994 Jan;11(2):349–361. doi: 10.1111/j.1365-2958.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Logan S. M., Kostrzynska M., Wadström T., Trust T. J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991 Jan;173(2):505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Taylor D. E. Comparison of methods used to separate the inner and outer membranes of cell envelopes of Campylobacter spp. J Gen Microbiol. 1988 Nov;134(11):2925–2932. doi: 10.1099/00221287-134-11-2925. [DOI] [PubMed] [Google Scholar]
- Poquet I., Kornacker M. G., Pugsley A. P. The role of the lipoprotein sorting signal (aspartate +2) in pullulanase secretion. Mol Microbiol. 1993 Sep;9(5):1061–1069. doi: 10.1111/j.1365-2958.1993.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Pridmore R. D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56(2-3):309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- Qian N., Sejnowski T. J. Predicting the secondary structure of globular proteins using neural network models. J Mol Biol. 1988 Aug 20;202(4):865–884. doi: 10.1016/0022-2836(88)90564-5. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993 Jul 20;232(2):584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987 Feb 11;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerbaum S., Josenhans C., Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993 Jun;175(11):3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Eaton M., Chang N., Salama S. M. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. J Bacteriol. 1992 Nov;174(21):6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Rioux C. R., Potter A. A. Molecular cloning, nucleotide sequence, and characterization of a 40,000-molecular-weight lipoprotein of Haemophilus somnus. Infect Immun. 1992 Mar;60(3):826–831. doi: 10.1128/iai.60.3.826-831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wu H. C. A distinct signal peptidase for prolipoprotein in Escherichia coli. J Cell Biochem. 1984;24(2):113–120. doi: 10.1002/jcb.240240203. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallich R., Simon M. M., Hofmann H., Moter S. E., Schaible U. E., Kramer M. D. Molecular and immunological characterization of a novel polymorphic lipoprotein of Borrelia burgdorferi. Infect Immun. 1993 Oct;61(10):4158–4166. doi: 10.1128/iai.61.10.4158-4166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Taylor D. E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990 Sep 28;94(1):23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Sequence of active site peptides from the penicillin-sensitive D-alanine carboxypeptidase of Bacillus subtilis. Mechanism of penicillin action and sequence homology to beta-lactamases. J Biol Chem. 1980 May 10;255(9):3964–3976. [PubMed] [Google Scholar]
- Weigel L. M., Brandt M. E., Norgard M. V. Analysis of the N-terminal region of the 47-kilodalton integral membrane lipoprotein of Treponema pallidum. Infect Immun. 1992 Apr;60(4):1568–1576. doi: 10.1128/iai.60.4.1568-1576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmüller K. H., Bessler W. G., Jung G. Solid phase peptide synthesis of lipopeptide vaccines eliciting epitope-specific B-, T-helper and T-killer cell response. Int J Pept Protein Res. 1992 Sep-Oct;40(3-4):255–260. doi: 10.1111/j.1399-3011.1992.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yu F., Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988 May 6;53(3):423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yao R., Alm R. A., Trust T. J., Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993 Aug 16;130(1):127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- Yu F., Inouye S., Inouye M. Lipoprotein-28, a cytoplasmic membrane lipoprotein from Escherichia coli. Cloning, DNA sequence, and expression of its gene. J Biol Chem. 1986 Feb 15;261(5):2284–2288. [PubMed] [Google Scholar]