Abstract
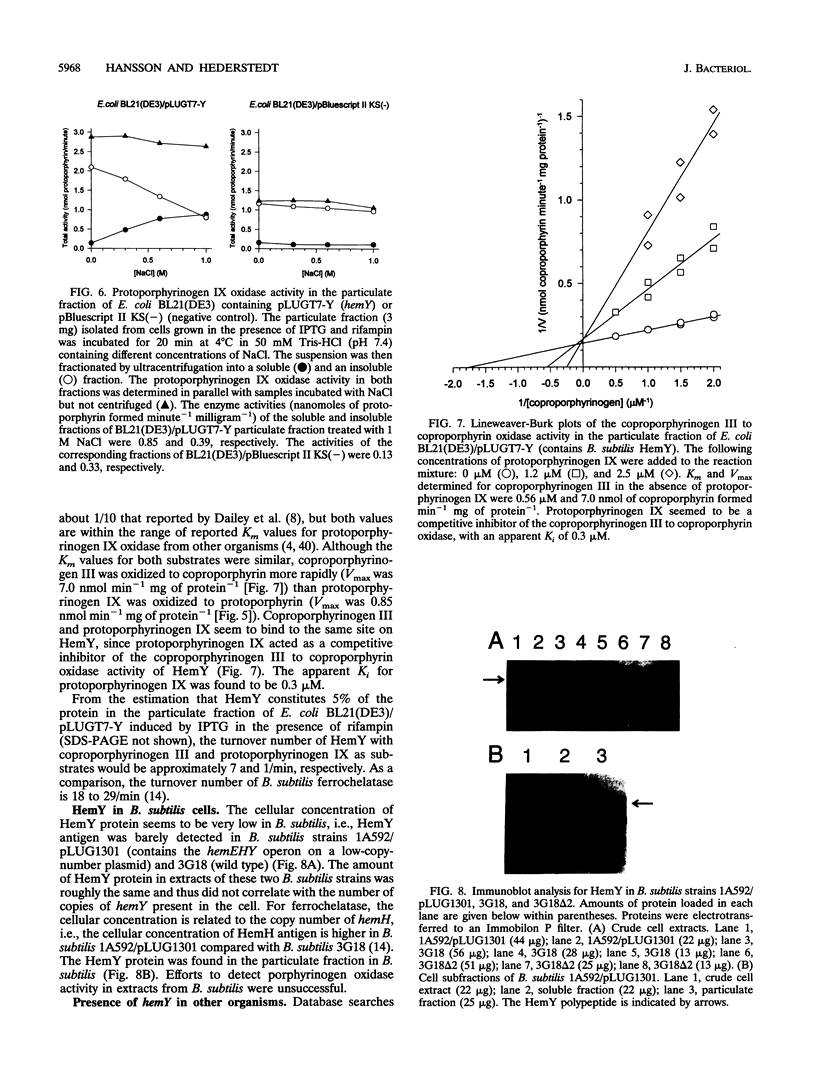
The hemY gene of the Bacillus subtilis hemEHY operon is essential for protoheme IX biosynthesis. Two previously isolated hemY mutations were sequenced. Both mutations are deletions affecting the hemY reading frame, and they cause the accumulation of coproporphyrinogen III or coproporphyrin III in the growth medium and the accumulation of trace amounts of other porphyrinogens or porphyrins intracellularly. HemY was found to be a 53-kDa peripheral membrane-bound protein. In agreement with recent findings by Dailey et al. (J. Biol. Chem. 269:813-815, 1994) B. subtilis HemY protein synthesized in Escherichia coli oxidized coproporphyrinogen III and protoporphyrinogen IX to coproporphyrin and protoporphyrin, respectively. The protein is not a general porphyrinogen oxidase since it did not oxidize uroporphyrinogen III. The apparent specificity constant, kcat/Km, for HemY was found to be about 12-fold higher with coproporphyrinogen III as a substrate compared with protoporphyrinogen IX as a substrate. The protoporphyrinogen IX oxidase activity is consistent with the function of HemY in a late step of protoheme IX biosynthesis, i.e., HemY catalyzes the penultimate step of the pathway. However, the efficient coproporphyrinogen III to coproporphyrin oxidase activity is unexplained in the current view of protoheme IX biosynthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek I., Miczák A., Ivánovics G. Mapping the delta-aminolaevulinic acid dehydrase and porphobilinogen deaminase loci in Bacillus subtilis. Mol Gen Genet. 1974;132(3):233–239. doi: 10.1007/BF00269396. [DOI] [PubMed] [Google Scholar]
- Berek I., Miczák A., Kiss I., Ivánovics G., Durkó I. Genetic and biochemical analysis of haemin dependent mutants of Bacillus subtilis. Acta Microbiol Acad Sci Hung. 1975;22(2):157–167. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Camadro J. M., Abraham N. G., Levere R. D. Kinetic properties of the membrane-bound human liver mitochondrial protoporphyrinogen oxidase. Arch Biochem Biophys. 1985 Oct;242(1):206–212. doi: 10.1016/0003-9861(85)90494-1. [DOI] [PubMed] [Google Scholar]
- Camadro J. M., Chambon H., Jolles J., Labbe P. Purification and properties of coproporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Eur J Biochem. 1986 May 2;156(3):579–587. doi: 10.1111/j.1432-1033.1986.tb09617.x. [DOI] [PubMed] [Google Scholar]
- Coomber S. A., Jones R. M., Jordan P. M., Hunter C. N. A putative anaerobic coproporphyrinogen III oxidase in Rhodobacter sphaeroides. I. Molecular cloning, transposon mutagenesis and sequence analysis of the gene. Mol Microbiol. 1992 Nov;6(21):3159–3169. doi: 10.1111/j.1365-2958.1992.tb01772.x. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Karr S. W. Purification and characterization of murine protoporphyrinogen oxidase. Biochemistry. 1987 May 19;26(10):2697–2701. doi: 10.1021/bi00384a007. [DOI] [PubMed] [Google Scholar]
- Dailey T. A., Meissner P., Dailey H. A. Expression of a cloned protoporphyrinogen oxidase. J Biol Chem. 1994 Jan 14;269(2):813–815. [PubMed] [Google Scholar]
- Daniels D. L., Plunkett G., 3rd, Burland V., Blattner F. R. Analysis of the Escherichia coli genome: DNA sequence of the region from 84.5 to 86.5 minutes. Science. 1992 Aug 7;257(5071):771–778. doi: 10.1126/science.1379743. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann H. C., Baldwin E. T. Reverse-phase purification and silica gel thin-layer chromatography of porphyrin carboxylic acids. Anal Biochem. 1984 Mar;137(2):473–480. doi: 10.1016/0003-2697(84)90115-5. [DOI] [PubMed] [Google Scholar]
- Hansson M., Hederstedt L. Cloning and characterization of the Bacillus subtilis hemEHY gene cluster, which encodes protoheme IX biosynthetic enzymes. J Bacteriol. 1992 Dec;174(24):8081–8093. doi: 10.1128/jb.174.24.8081-8093.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Hederstedt L. Purification and characterisation of a water-soluble ferrochelatase from Bacillus subtilis. Eur J Biochem. 1994 Feb 15;220(1):201–208. doi: 10.1111/j.1432-1033.1994.tb18615.x. [DOI] [PubMed] [Google Scholar]
- Hansson M., Rutberg L., Schröder I., Hederstedt L. The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol. 1991 Apr;173(8):2590–2599. doi: 10.1128/jb.173.8.2590-2599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss I., Berek I., Ivánovics G. Mapping the -aminolaevulinic acid synthetase locus in Bacillus subtilis. J Gen Microbiol. 1971 May;66(2):153–159. doi: 10.1099/00221287-66-2-153. [DOI] [PubMed] [Google Scholar]
- Klemm D. J., Barton L. L. Purification and properties of protoporphyrinogen oxidase from an anaerobic bacterium, Desulfovibrio gigas. J Bacteriol. 1987 Nov;169(11):5209–5215. doi: 10.1128/jb.169.11.5209-5215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H., Furukawa T., Yoshinaga T., Tokunaga R., Taketani S. Coproporphyrinogen oxidase. Purification, molecular cloning, and induction of mRNA during erythroid differentiation. J Biol Chem. 1993 Oct 5;268(28):21359–21363. [PubMed] [Google Scholar]
- Li S., Lee B. U., Shimkets L. J. csgA expression entrains Myxococcus xanthus development. Genes Dev. 1992 Mar;6(3):401–410. doi: 10.1101/gad.6.3.401. [DOI] [PubMed] [Google Scholar]
- Madsen O., Sandal L., Sandal N. N., Marcker K. A. A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules. Plant Mol Biol. 1993 Oct;23(1):35–43. doi: 10.1007/BF00021417. [DOI] [PubMed] [Google Scholar]
- Martasek P., Camadro J. M., Delfau-Larue M. H., Dumas J. B., Montagne J. J., de Verneuil H., Labbe P., Grandchamp B. Molecular cloning, sequencing, and functional expression of a cDNA encoding human coproporphyrinogen oxidase. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3024–3028. doi: 10.1073/pnas.91.8.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczák A., Berek I., Ivanovics G. Mapping the uroporphyrinogen decarboxylase, coproporphyrinogen oxidase and ferrochelatase loci in Bacillus subtilis. Mol Gen Genet. 1976 Jul 5;146(1):85–87. doi: 10.1007/BF00267986. [DOI] [PubMed] [Google Scholar]
- Miczák A., Prágai B., Berek I. Mapping the uroporphyrinogen III cosynthase locus in Bacillus subtilis. Mol Gen Genet. 1979 Jul 24;174(3):293–295. doi: 10.1007/BF00267802. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K., Inokuchi H. Nucleotide sequence between the fadB gene and the rrnA operon from Escherichia coli. Nucleic Acids Res. 1990 Nov 11;18(21):6439–6439. doi: 10.1093/nar/18.21.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Niaudet B., Goze A., Ehrlich S. D. Insertional mutagenesis in Bacillus subtilis: mechanism and use in gene cloning. Gene. 1982 Oct;19(3):277–284. doi: 10.1016/0378-1119(82)90017-8. [DOI] [PubMed] [Google Scholar]
- Petricek M., Rutberg L., Schröder I., Hederstedt L. Cloning and characterization of the hemA region of the Bacillus subtilis chromosome. J Bacteriol. 1990 May;172(5):2250–2258. doi: 10.1128/jb.172.5.2250-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett G., 3rd, Burland V., Daniels D. L., Blattner F. R. Analysis of the Escherichia coli genome. III. DNA sequence of the region from 87.2 to 89.2 minutes. Nucleic Acids Res. 1993 Jul 25;21(15):3391–3398. doi: 10.1093/nar/21.15.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K. L., Dailey H. A. Characteristics of murine protoporphyrinogen oxidase. Protein Sci. 1992 Jun;1(6):801–809. doi: 10.1002/pro.5560010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseier T. M., Winteler H. V., Hennecke H. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem. 1991 Apr 25;266(12):7793–7803. [PubMed] [Google Scholar]
- Rondahl H., Nilsson B., Holmgren E. Fusions to the 5' end of a gene encoding a two-domain analogue of staphylococcal protein A. J Biotechnol. 1992 Sep;25(3):269–287. doi: 10.1016/0168-1656(92)90161-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Letowski J., Czaika G., Ramirez V., Nead M. A., Jacobs J. M., Morais R. Nucleotide sequence of the hemG gene involved in the protoporphyrinogen oxidase activity of Escherichia coli K12. Can J Microbiol. 1993 Dec;39(12):1155–1161. doi: 10.1139/m93-174. [DOI] [PubMed] [Google Scholar]
- Savioz A., Zimmermann A., Haas D. Pseudomonas aeruginosa promoters which contain a conserved GG-N10-GC motif but appear to be RpoN-independent. Mol Gen Genet. 1993 Apr;238(1-2):74–80. doi: 10.1007/BF00279533. [DOI] [PubMed] [Google Scholar]
- Siepker L. J., Ford M., de Kock R., Kramer S. Purification of bovine protoporphyrinogen oxidase: immunological cross-reactivity and structural relationship to ferrochelatase. Biochim Biophys Acta. 1987 Jul 7;913(3):349–358. doi: 10.1016/0167-4838(87)90146-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troup B., Jahn M., Hungerer C., Jahn D. Isolation of the hemF operon containing the gene for the Escherichia coli aerobic coproporphyrinogen III oxidase by in vivo complementation of a yeast HEM13 mutant. J Bacteriol. 1994 Feb;176(3):673–680. doi: 10.1128/jb.176.3.673-680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Xu K., Delling J., Elliott T. The genes required for heme synthesis in Salmonella typhimurium include those encoding alternative functions for aerobic and anaerobic coproporphyrinogen oxidation. J Bacteriol. 1992 Jun;174(12):3953–3963. doi: 10.1128/jb.174.12.3953-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Elliott T. An oxygen-dependent coproporphyrinogen oxidase encoded by the hemF gene of Salmonella typhimurium. J Bacteriol. 1993 Aug;175(16):4990–4999. doi: 10.1128/jb.175.16.4990-4999.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Elliott T. Cloning, DNA sequence, and complementation analysis of the Salmonella typhimurium hemN gene encoding a putative oxygen-independent coproporphyrinogen III oxidase. J Bacteriol. 1994 Jun;176(11):3196–3203. doi: 10.1128/jb.176.11.3196-3203.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sano S. Coproporphyrinogen oxidase. I. Purification, properties, and activation by phospholipids. J Biol Chem. 1980 May 25;255(10):4722–4726. [PubMed] [Google Scholar]
- Zagorec M., Buhler J. M., Treich I., Keng T., Guarente L., Labbe-Bois R. Isolation, sequence, and regulation by oxygen of the yeast HEM13 gene coding for coproporphyrinogen oxidase. J Biol Chem. 1988 Jul 15;263(20):9718–9724. [PubMed] [Google Scholar]