Abstract
Leptin-deficient ob/ob mice exhibit several metabolic and immune abnormalities, including thymus atrophy and markedly reduced inflammatory responses. We evaluated whether transplantation of wild type (WT) white adipose tissue (WAT) into ob/ob mice could mimic the effect of recombinant leptin administration in normalizing metabolic, immune and inflammatory abnormalities. Female ob/ob mice received a subcutaneous transplantation of WAT obtained from WT littermates. A separate group of ob/ob mice was sham-operated. Despite raising leptin levels to only 15% of those observed in WT mice, WAT transplantation normalized metabolic abnormalities (glycemia, ALT, liver weight) in ob/ob mice and prevented further body weight gain. The transplanted group demonstrated normalization of thymus and spleen cellularity, thymocyte subpopulations and rates of thymocyte apoptosis. In the model of dextran sulfate sodium-induced colitis, WAT transplantation restored inflammation to levels equivalent to those of WT mice. Colonic production of IL-6 and MIP-2 was markedly reduced in the non-transplanted ob/ob group compared to transplanted ob/ob and WT mice. Our data indicate that WAT transplantation is an effective way to normalize metabolic as well as immune and inflammatory parameters in ob/ob mice. The threshold of leptin sufficient to normalize metabolic, immune and inflammatory function is significantly lower than levels present in lean WT mice. Finally, leptin derived exclusively from WAT is sufficient to normalize metabolic, immune and inflammatory parameters in ob/ob mice.
Introduction
Leptin, a 16 kDa protein encoded by the ob gene, is a pleiotropic hormone which regulates appetite and metabolism as well as immunity and inflammation [1]. In addition to being massively obese and infertile, leptin-deficient ob/ob mice exhibit several metabolic alterations, including hepatomegaly with steatosis and increased transaminase levels as well as hyperglycemia and insulin resistance [2]. Leptin deficiency is also associated with immune and inflammatory abnormalities. In fact, decreased thymus cellularity accompanied by increased apoptosis of CD4+ CD8+ immature thymocytes, resistance to several experimental models of inflammation/autoimmunity and altered production of pro-inflammatory cytokines are observed in ob/ob mice [3–6]. In particular, ob/ob mice exhibit markedly reduced disease severity in the experimental model of intestinal inflammation induced by administration of the irritant dextran sodium sulfate (DSS), whereas reconstitution with exogenous leptin restores their response [6].
Recently, transplantation of white adipose tissue (WAT) has been reported to effectively and protractedly reduce body weight and food intake, reduce insulin, glucose and corticosterone levels and restore fertility in ob/ob mice, despite the fact that circulating leptin levels reached at best 20% of WT levels in transplanted mice [7], thus supporting the concept of a threshold level for leptin in regulating neuroendocrine and metabolic function. However, it is currently unclear if such a threshold effect is also important for regulation of immune responses by leptin.
Quantitatively, adipocytes are the most important source of leptin [2]. However, leptin is also produced - albeit at much reduced levels compared to adipocytes - by other tissues and cell types, including gastric fundic mucosa, inflamed colonic epithelium as well as activated T lymphocytes [8–11]. The function of non adipocyte-derived leptin in regulating metabolic and inflammatory response has not been completely characterized, although cell transfer experiments indicate lack of a significant role for T cell-derived leptin in hepatic and intestinal inflammation [12].
In the present study, we evaluated the effect of WT WAT transplantation on body weight, metabolic, immune and inflammatory parameters in ob/ob mice. Our results confirm previous data indicating that transplantation of a small amount (∼ 1g) of WT WAT is sufficient to stop body weight gain and normalize glucose levels in ob/ob mice [7]. We also demonstrate for the first time that WAT transplantation restores thymus and spleen cellularity and the intestinal inflammatory response in ob/ob mice.
Materials and Methods
WAT transplantation
Animal protocols were approved by the Animal Studies Committee of the University of Illinois at Chicago. Six-week-old female leptin-deficient (B6.V-Lepob/J, referred to as ob/ob) mice and their lean littermates were obtained from The Jackson Laboratory. A group of ob/ob mice received WAT transplantation as described in [13]. Briefly, gonadal WAT from WT littermates euthanized by cervical dislocation was placed in sterile PBS and cut into small pieces. The grafts were implanted subcutaneously through small incisions in the shaved skin of the back of recipient ob/ob mice anesthetized with isoflurane. Approximately 1 g of tissue was transplanted per mouse. Incisions were closed using wound clips and mice observed for wound closure. A second group of ob/ob mice was sham-operated. Mice were weighed twice weekly, beginning one day before transplant. A group of WT mice was also weighed and observed for comparison. Mice were observed for 6 weeks after transplant, at which point they were sacrificed by cervical dislocation under isoflurane anesthesia immediately after obtaining blood from the retroorbital plexus. Liver, thymus and spleen were obtained and processed immediately after euthanasia.
DSS-induced colitis
DSS colitis was induced and evaluated as previously described [6], with minor modifications. Beginning on day 47 after WAT transplant, mice were fed 2% DSS (mol wt, 40 kD; ICN, Aurora, OH) dissolved in sterile water ad libitum for 5 days followed by 5 days of normal drinking water. Control mice were fed tap water (without DSS). Mice were weighed daily and monitored for appearance of diarrhea and blood in the stools. A previously described clinical score which takes into account body weight loss, diarrhea and blood in the stools was used to quantify disease [14]. At the end of the experiment, colon cultures were performed as previously described [6]. Culture supernatants were used to evaluated production of IL-6 and macrophage inflammatory protein-2 (MIP-2) by specific ELISA (R&D Systems, Minneapolis, MN).
Thymocyte and splenocyte isolation and flow cytometry
Thymocytes and splenocytes were freshly isolated using a 100μm cell strainer (Fisher Scientific, Pittsburgh, PA). Cells were suspended in complete cell culture medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin; Invitrogen Life Technologies, Carlsbad, CA), washed once and counted. For CD4 and CD8 T cell staining, thymocytes (106) were incubated with 1 μg of FITC-conjugated anti-CD4 and 1 μg of PE-conjugated anti-CD8 (BD Pharmingen, San Diego, CA) for 20 minutes at 4° C and washed with cold PBS three times. Rat IgG conjugated with FITC and PE served as negative control. Cells were then analyzed using a FACS Calibur (Becton Dickinson, San Diego, CA).
Miscellaneous measurements
Leptin, adiponectin, resistin, IL-6 and MIP-2 levels were measured using specific ELISA kits (R&D Systems). ALT levels were measured using a kit from Teco Diagnostics (Anaheim, CA). Glucose levels were measured using a glucometer (Bayer, Leverkusen Germany).
Statistical analysis
Data are expressed as mean +/− SEM. Statistical significance of differences between treatment and control groups were determined by factorial ANOVA. Statistical analyses were performed using the XLStat software (Addinsoft, Brooklyn, NY).
Results and Discussion
Effect of WAT transplantation on body weight, metabolic parameters and adipokine levels
Transplantation of WT WAT effectively reduced adiposity (Fig. 1) and normalized the pronounced hepatomegaly, increased serum ALT and glucose levels characteristically observed in ob/ob mice (Fig. 2), in agreement with a previous report [7]. Interestingly, reduced adiposity and normalization of metabolic alterations were observed even in the presence of sharply reduced leptin levels in transplanted ob/ob compared to WT mice (Table 1). In fact, serum leptin in WAT transplanted ob/ob mice reached only 15% of the levels observed in WT mice, a result in agreement with data published by Klebanov et al [7]. The observation that very low circulating levels of leptin were sufficient to significantly ameliorate the phenotype of ob/ob mice has two possible explanations, not necessarily mutually exclusive: 1) ob/ob mice are exquisitely sensitive to leptin, as also suggested by their response to administration of low levels of recombinant leptin [15]. This increased sensitivity is likely due to overexpression of leptin receptors [16]; 2) increasing leptin levels above a low, critical threshold is not necessary for this adipokine to exert its full biological activity and higher levels may actually reduce sensitivity and lead to development of leptin resistance, as suggested by studies evaluating the effect of short-term fasting on hormonal and immune parameters in humans [17].
Figure 1. Effect of WAT transplantation on body weight.
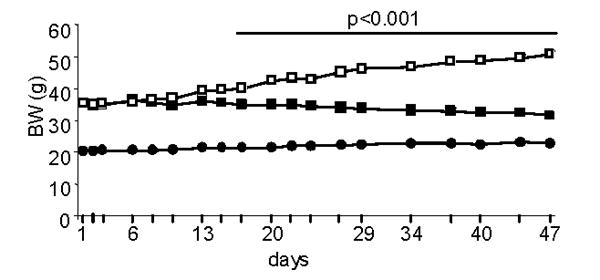
Body weight was measured in ob/ob mice transplanted with WAT (closed squares), sham-operated ob/ob mice (open squares) and WT mice (closed circles) starting on the day of WAT transplant and continuing until day 47. Data are mean +/− SEM of 10 mice per group. BW of ob/ob sham was significantly different from that ob/ob transplant starting on day 17 and continuing until day 47.
Figure 2. Effect of WAT transplantation on liver weight, ALT and glucose levels.
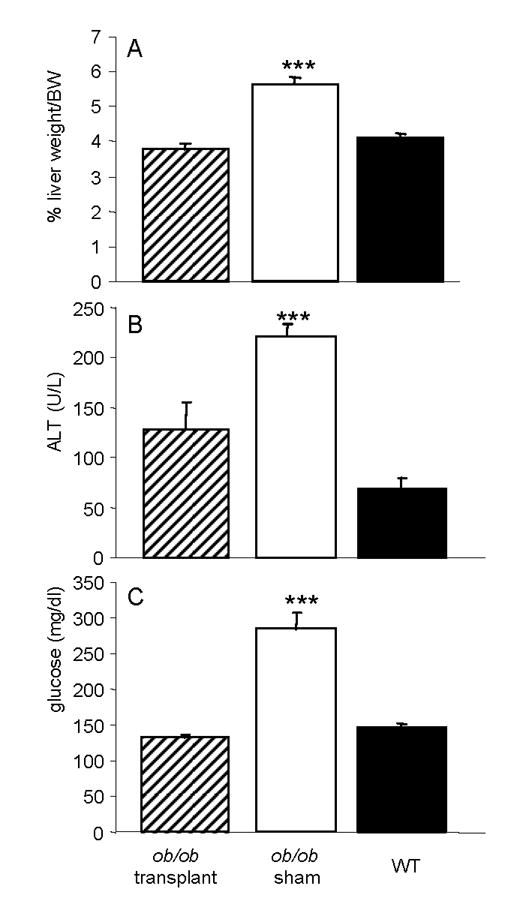
Liver and serum were obtained from ob/ob mice transplanted with WAT (hatched columns), sham-operated ob/ob mice (open columns) and WT mice (closed columns) on day 47. Panel A: liver weight as percent of BW; Panel B: Serum ALT levels; Panel C: blood glucose levels. Data are mean +/− SEM of 10 mice per group. *** p<0.001 versus ob/ob transplant and WT.
Table 1.
Effect of WAT transplantation on circulating adipokine levels
| Leptin (ng/ml) | Adiponectin (μg/ml) | Resistin (ng/ml) | |
|---|---|---|---|
| ob/ob transplant | 0.60 +/− 0.10 *** | 8.11 +/− 0.72* | 2.51 +/− 0.09* |
| ob/ob sham | <0.02 | 7.58 +/− 0.51* | 2.63 +/− 0.07* |
| WT | 4.10 +/− 0.99 | 6.95 +/− 0.63 | 2.11 +/− 0.14 |
Blood was obtained on day 47 from ob/ob transplant, ob/ob sham and WT mice and serum prepared for measurement of leptin, adiponectin and resistin by ELISA. Data are mean +/− SEM of 10 mice per group. *p<0.05, *** p<0.001 versus WT.
In contrast to leptin, serum levels of adiponectin and resistin in ob/ob mice were not significantly altered by WAT transplantation (Table 2). Serum adiponectin and resistin levels were slightly but significantly higher in both sham and transplanted ob/ob compared to WT mice, in agreement with some [18–20] though not other [21, 22] previously published data. The reasons for the above-mentioned discrepancies in circulating adiponectin and resistin levels in ob/ob mice are currently unclear and may be secondary to experimental differences such as age, gender and genetic background of the mice studied by different investigators.
Table 2.
Effect of WAT transplantation on thymus and spleen cellularity
| Thymocytes (106) | Splenocytes (106) | |
|---|---|---|
| ob/ob transplant | 118.67 +/− 32.47 | 189.33 +/− 11.02 |
| ob/ob sham | 66.75 +/− 14.52 ** | 138.50 +/− 18.45 * |
| WT | 124.40 +/− 5.08 | 186.00 +/− 19.32 |
Thymi and spleens were obtained on day 47 from ob/ob transplant, ob/ob sham and WT mice and cellularity evaluated. Data are mean +/− SEM of 5 mice per group. * p<0.05, ** p<0.01 versus WT and ob/ob transplant.
Effect of WAT transplantation on thymus and spleen cellularity
Several alterations of the immune system are present in ob/ob mice and are normalized by leptin administration, including thymus atrophy and reduced cellularity of the spleen [3, 23]. Although leptin is mostly produced by white adipocytes, activated T lymphocytes - in addition to other cell types - also produce this adipokine [10]. Since ob/ob mice lack production of leptin from every cell type and administration of recombinant leptin unspecifically raises systemic leptin levels, it has until now not been possible to discriminate the cellular source of leptin necessary for modulation of the immune system.
Transplantation of WT WAT completely reversed thymus atrophy as well as reduced spleen cellularity in ob/ob mice (Table 2) and normalized thymocyte subpopulations, increasing the number of immature CD4+ CD8+ and reducing CD4− CD8+ thymocytes (Fig. 3), an effect comparable to that achieved by administration of recombinant leptin [3]. Since in WAT-transplanted ob/ob mice leptin is exclusively produced by the transplanted tissue, these data clearly demonstrate that WAT-derived leptin is sufficient to maintain normal thymus and spleen cellularity and that lymphocyte-derived leptin is not necessary for this process, although we cannot exclude that leptin produced by lymphocytes might play a role under normal physiological conditions. Furthermore, the same remarks mentioned above concerning leptin threshold effects as well leptin sensitivity in ob/ob mice apply not only to the regulation of appetite and metabolism by leptin, but also to its effects on immunity.
Figure 3. Effect of WAT transplantation on thymocyte subpopulations.
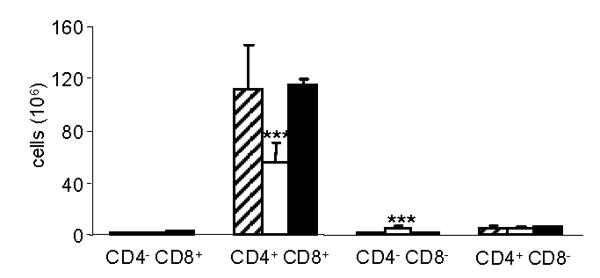
Thymocytes were isolated from the thymus of ob/ob mice transplanted with WAT (hatched columns), sham-operated ob/ob mice (open columns) and WT mice (closed columns) and analyzed by flow cytometry as described in the Methods section. Data are mean +/− SEM of 5 mice per group. *** p<0.001 versus ob/ob transplant and WT.
Effect of WAT transplantation on DSS-induced intestinal inflammation
In addition to reduced cellularity of immune organs, ob/ob mice also develop significantly reduced inflammatory responses in a variety of experimental models [3–6]. In particular, leptin deficiency is associated with a markedly impaired colonic inflammatory response following administration of the irritant DSS [6]. Using cell transfer experiments, we have previously demonstrated that leptin produced by activated T lymphocytes does not play a critical role in mediating colonic inflammation in the model of colitis induced by transfer of CD4+ CD45RBhigh cells into scid mice [12]. However, activated T lymphocytes are not the only cells able to produce leptin in the inflamed intestine, since colonic epithelial cells activated by inflammatory stimuli can express and release leptin [9]. The contribution of WAT- versus colon-derived leptin in modulating intestinal inflammation is currently unclear.
As shown in Fig. 4, transplantation of WT WAT into ob/ob mice completely reversed the impaired inflammatory response to DSS, as evaluated by a clinical score that include measurements of body weight loss, diarrhea and presence of blood in the stools. Whereas sham-operated ob/ob mice developed only a very mild disease, the clinical scores of WT and transplanted ob/ob mice were not significantly different from each other at any of the time points examined. Colon shortening is a typical manifestation of inflammation in response to DSS [24]. Whereas sham-operated ob/ob mice did not demonstrate any significant shortening in the colon after DSS administration, marked shortening occurred in both WT and transplanted ob/ob mice (Table 3), further confirming the effectiveness of WAT transplantation in restoring colonic inflammation in ob/ob mice. Finally, production of the pro-inflammatory cytokines IL-6 and MIP-2 was significantly lower in colon cultures obtained from sham-operated ob/ob mice as compared to cultures obtained from WT or transplanted ob/ob mice (Fig. 5). Taken together, these data indicate that WAT-derived leptin is sufficient to restore colonic inflammation in ob/ob mice, whereas colon-derived leptin is not necessary.
Figure 4. DSS-induced intestinal inflammation: effect of WAT transplantation.
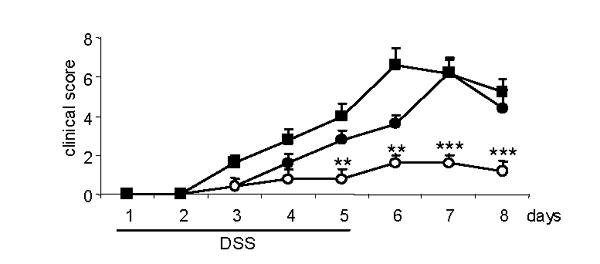
ob/ob mice transplanted with WAT (closed squares), sham-operated ob/ob mice (open circles) and WT mice (closed circles) received DSS for 5 days following the schedule described in the Methods section. Clinical score was evaluated daily. Data are mean +/− SEM of 5 mice per group. ** p<0.01, *** p<0.001 versus ob/ob transplant and WT.
Table 3.
Effect of WAT transplantation on DSS-induced colon shortening
| Water | DSS | |
|---|---|---|
| ob/ob transplant | 7.75 +/− 0.10 | 5.54 +/− 0.31** |
| ob/ob sham | 7.88 +/− 0.27 | 7.14 +/− 0.26 |
| WT | 7.18 +/− 0.11 | 4.96 +/− 0.19** |
Mice received either regular drinking water or DSS as detailed in the Methods sections. On day 10, colons were removed and their length measured using a ruler. Data are expressed as cm of colon length. Data are mean +/− SEM of 5 mice per group. ** p<0.01 versus respective water.
Figure 5. Effect of WAT transplantation on colonic production of IL-6 and MIP-2.
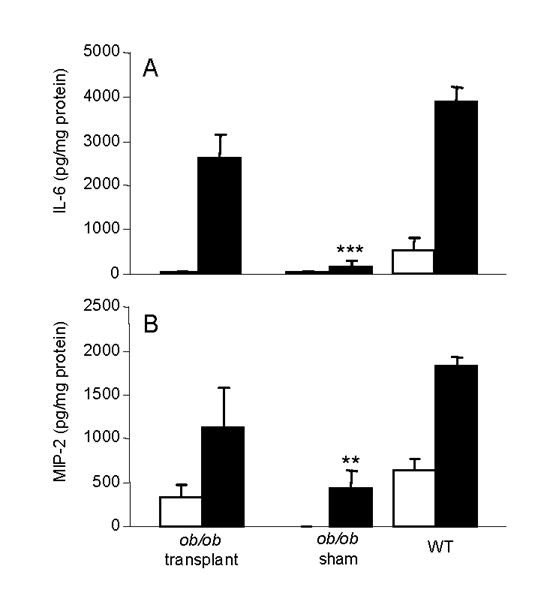
On day 8 of administration of either regular drinking water (open columns) or DSS (closed colums), colon cultures were performed and levels of IL-6 (panel A) and MIP-2 (panel B) evaluated in the supernatants. Data are mean +/− SEM of 5 mice per group. ** p<0.01, *** p<0.001 versus ob/ob transplant and WT treated with DSS.
Conclusions
The present report demonstrates that leptin derived exclusively from white adipose tissue is sufficient to reverse the metabolic, immune and inflammatory alterations of ob/ob mice. Although our data do not exclude the possibility that local production of leptin in immune organs and in the inflammatory microenvironment might further modulate immunity and inflammation, the results clearly indicate that local leptin production is not necessary to maintain normal thymus and spleen cellularity and to allow for a full development of colonic inflammation.
Acknowledgments
This work was supported by NIH grants DK61483 and DK068035 (to GF).
Footnotes
Disclosure statement: The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60:S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 3.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor-a and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, et al. Requirement for leptin in the induction of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 5.Busso N, So A, Chobaz-Peclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 6.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 7.Klebanov S, Astle CM, DeSimone O, Ablamunitis V, Harrison DE. Adipose tissue transplantation protects ob/ob mice from obesity, restores insulin sensitivity and restores fertility. J Endocrinol. 2005;186:203–211. doi: 10.1677/joe.1.06150. [DOI] [PubMed] [Google Scholar]
- 8.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 9.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, et al. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. Faseb J. 2004;18:696–698. doi: 10.1096/fj.03-0422fje. [DOI] [PubMed] [Google Scholar]
- 10.Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegmund B, Sennello JA, Lehr HA, Batra A, Fedke I, Zeitz M, et al. Development of intestinal inflammation in double IL-10- and leptin-deficient mice. J Leukoc Biol. 2004;76:782–786. doi: 10.1189/jlb.0404239. [DOI] [PubMed] [Google Scholar]
- 12.Fantuzzi G, Sennello JA, Batra A, Fedke I, Lehr AH, Zeitz M, et al. Defining the role of T cell-derived leptin in the modulation of hepatic or intestinal inflammation in mice. Clin Exp Immunol. 2005;142:31–38. doi: 10.1111/j.1365-2249.2005.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegmund B, Sennello JA, Jones-Carson J, Gamboni-Robertson F, Lehr HA, Batra A, et al. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:965–969. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montez JM, Soukas AA, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JE. Acute leptin deficiency, leptin resistance and the physiologic response to leptin withdrawal. Proc Nat Acad Sci USA. 2005 doi: 10.1073/pnas.0409530102. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, et al. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypresensitivity and fasting. Diabetes. 1998;47:538–543. doi: 10.2337/diabetes.47.4.538. [DOI] [PubMed] [Google Scholar]
- 17.Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Nat Acad Sci USA. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haluzik M, Dietz KR, Kim JK, Marcus-Samuels B, Shulman GI, Gavrilova O, et al. Adrenalectomy improves diabetes in A-ZIP/F-1 lipoatrophic mice by increasing both liver and muscle insulin sensitivity. Diabetes. 2002;51:2113–2118. doi: 10.2337/diabetes.51.7.2113. [DOI] [PubMed] [Google Scholar]
- 19.Delporte ML, El Mkadem SA, Quisquater M, Brichard SM. Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. Am J Physiol Endocrinol Metab. 2004;287:E446–E453. doi: 10.1152/ajpendo.00488.2003. [DOI] [PubMed] [Google Scholar]
- 20.Sennello JA, Fayad R, Morris AM, Eckel RH, Asilmaz E, Montez JM, et al. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology. 2005;146:2157–2164. doi: 10.1210/en.2004-1572. [DOI] [PubMed] [Google Scholar]
- 21.Fujinami A, Ohta K, Matsui H, Kitada N, Kitaura Y, Kawahara Y, et al. Resistin concentrations in murine adipose tissue and serum measured y a new enzyme immunoassay. Obesity (Silver Spring) 2006;14:199–205. doi: 10.1038/oby.2006.26. [DOI] [PubMed] [Google Scholar]
- 22.Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am J Physiol Endocrinol Metab. 2002;283:E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- 23.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 24.Okayasu I, Hatakeyama S, Yamada M, Okhusa T, Inagaki Y, Nakaya R. A novel method for the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]


