Abstract
Thrust for propulsion of flagellated bacteria is generated by rotation of a propeller, the flagellum. The power to drive the polar flagellar rotary motor of Vibrio parahaemolyticus is derived from the transmembrane potential of sodium ions. Force is generated by the motor on coupling of the movement of ions across the membrane to rotation of the flagellum. A gene, motX, encoding one component of the torque generator has been cloned and sequenced. The deduced protein sequence is 212 amino acids in length. MotX was localized to the membrane and shown to interact with MotY, which is the presumed stationary component of the motor. Overproduction of MotX, but not that of a nonfunctional mutant MotX, was lethal to Escherichia coli. The rate of lysis caused by induction of motX was proportional to the sodium ion concentration. Li+ and K+ substituted for Na+ to promote lysis, while Ca2+ did not enhance lysis. Protection from the lethal effects of induction of motX was afforded by the sodium channel blocker amiloride. The data suggest that MotX forms a sodium channel. The deduced protein sequence for MotX shows no homology to its ion-conducting counterpart in the proton-driven motor; however, in possessing only one hydrophobic domain, it resembles other channels formed by small proteins with single membrane-spanning domains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi T., McCarter L., Imae Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature. 1992 Jan 9;355(6356):182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- Barbry P., Chassande O., Duval D., Rousseau B., Frelin C., Lazdunski M. Biochemical identification of two types of phenamil binding sites associated with amiloride-sensitive Na+ channels. Biochemistry. 1989 May 2;28(9):3744–3749. doi: 10.1021/bi00435a018. [DOI] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Torque generated by the flagellar motor of Escherichia coli. Biophys J. 1993 Nov;65(5):2201–2216. doi: 10.1016/S0006-3495(93)81278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. F., Berg H. C. Mutations in the MotA protein of Escherichia coli reveal domains critical for proton conduction. J Mol Biol. 1991 Oct 20;221(4):1433–1442. doi: 10.1016/0022-2836(91)90943-z. [DOI] [PubMed] [Google Scholar]
- Blair D. F., Berg H. C. Restoration of torque in defective flagellar motors. Science. 1988 Dec 23;242(4886):1678–1681. doi: 10.1126/science.2849208. [DOI] [PubMed] [Google Scholar]
- Blair D. F., Berg H. C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell. 1990 Feb 9;60(3):439–449. doi: 10.1016/0092-8674(90)90595-6. [DOI] [PubMed] [Google Scholar]
- Chun S. Y., Parkinson J. S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988 Jan 15;239(4837):276–278. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Cohen F. S., Merrill A. R., Song H. Y. Structure and dynamics of the colicin E1 channel. Mol Microbiol. 1990 Apr;4(4):519–526. doi: 10.1111/j.1365-2958.1990.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Dean G. E., Macnab R. M., Stader J., Matsumura P., Burks C. Gene sequence and predicted amino acid sequence of the motA protein, a membrane-associated protein required for flagellar rotation in Escherichia coli. J Bacteriol. 1984 Sep;159(3):991–999. doi: 10.1128/jb.159.3.991-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Folander K., Smith J. S., Antanavage J., Bennett C., Stein R. B., Swanson R. Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron. 1991 Sep;7(3):403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Barondess J., Manoil C., Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J Mol Biol. 1987 May 20;195(2):289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
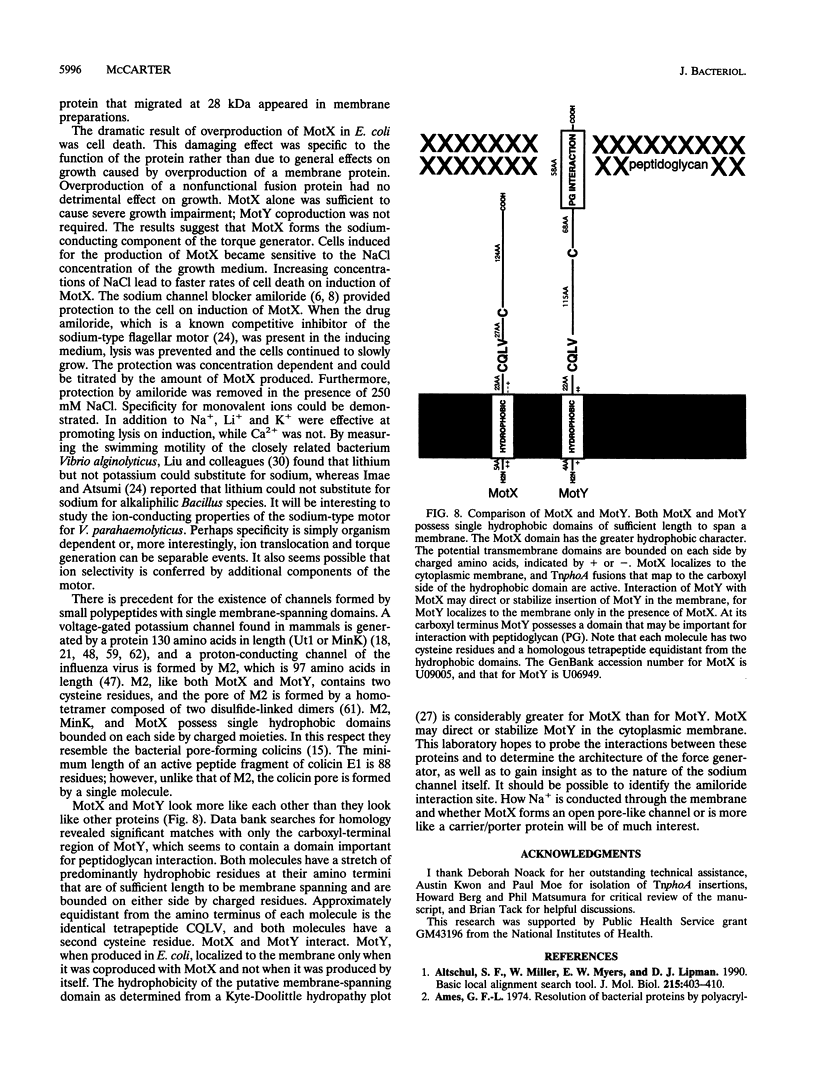
- Helmann J. D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991 Dec;5(12):2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Imae Y., Atsumi T. Na+-driven bacterial flagellar motors. J Bioenerg Biomembr. 1989 Dec;21(6):705–716. doi: 10.1007/BF00762688. [DOI] [PubMed] [Google Scholar]
- Imae Y., Matsukura H., Kobayasi S. Sodium-driven flagellar motors of alkalophilic Bacillus. Methods Enzymol. 1986;125:582–592. doi: 10.1016/s0076-6879(86)25047-8. [DOI] [PubMed] [Google Scholar]
- Khan S., Dapice M., Reese T. S. Effects of mot gene expression on the structure of the flagellar motor. J Mol Biol. 1988 Aug 5;202(3):575–584. doi: 10.1016/0022-2836(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Z., Dapice M., Khan S. Ion selectivity of the Vibrio alginolyticus flagellar motor. J Bacteriol. 1990 Sep;172(9):5236–5244. doi: 10.1128/jb.172.9.5236-5244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Parkinson J. S. Genetic analysis of the bacterial flagellum. Trends Genet. 1991 Jun;7(6):196–200. doi: 10.1016/0168-9525(91)90436-t. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Proton-driven bacterial flagellar motor. Methods Enzymol. 1986;125:563–581. doi: 10.1016/s0076-6879(86)25046-6. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Showalter R., Silverman M. Identification of a locus controlling expression of luminescence genes in Vibrio harveyi. J Bacteriol. 1989 May;171(5):2406–2414. doi: 10.1128/jb.171.5.2406-2414.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L. MotY, a component of the sodium-type flagellar motor. J Bacteriol. 1994 Jul;176(14):4219–4225. doi: 10.1128/jb.176.14.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L., Silverman M. Phosphate regulation of gene expression in Vibrio parahaemolyticus. J Bacteriol. 1987 Aug;169(8):3441–3449. doi: 10.1128/jb.169.8.3441-3449.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L., Wright M. E. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J Bacteriol. 1993 Jun;175(11):3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L., Hilmen M., Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988 Jul 29;54(3):345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- McCarter L., Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990 Jul;4(7):1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Meister M., Lowe G., Berg H. C. The proton flux through the bacterial flagellar motor. Cell. 1987 Jun 5;49(5):643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mirel D. B., Lustre V. M., Chamberlin M. J. An operon of Bacillus subtilis motility genes transcribed by the sigma D form of RNA polymerase. J Bacteriol. 1992 Jul;174(13):4197–4204. doi: 10.1128/jb.174.13.4197-4204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J. A., Innis M. A., Koonin E. V., Rudd K. E., Banuett F., Herskowitz I. The Escherichia coli hflA locus encodes a putative GTP-binding protein and two membrane proteins, one of which contains a protease-like domain. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10866–10870. doi: 10.1073/pnas.90.22.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner E., Kotler Y., Padan E., Schuldiner S. Physiological role of nhaB, a specific Na+/H+ antiporter in Escherichia coli. J Biol Chem. 1993 Jan 25;268(3):1729–1734. [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Pragnell M., Snay K. J., Trimmer J. S., MacLusky N. J., Naftolin F., Kaczmarek L. K., Boyle M. B. Estrogen induction of a small, putative K+ channel mRNA in rat uterus. Neuron. 1990 May;4(5):807–812. doi: 10.1016/0896-6273(90)90207-v. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar N., McCarter L., Simon M., Silverman M. Chemotactic control of the two flagellar systems of Vibrio parahaemolyticus. J Bacteriol. 1990 Jan;172(1):334–341. doi: 10.1128/jb.172.1.334-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H. P. The pUC18CM plasmids: a chloramphenicol resistance gene cassette for site-directed insertion and deletion mutagenesis in Escherichia coli. Biotechniques. 1990 Jun;8(6):612-3, 616. [PubMed] [Google Scholar]
- Silverman M., Showalter R., McCarter L. Genetic analysis in vibrio. Methods Enzymol. 1991;204:515–536. doi: 10.1016/0076-6879(91)04026-k. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Operon controlling motility and chemotoxis in E. coli. Nature. 1976 Dec 9;264(5586):577–580. doi: 10.1038/264577a0. [DOI] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stader J., Matsumura P., Vacante D., Dean G. E., Macnab R. M. Nucleotide sequence of the Escherichia coli motB gene and site-limited incorporation of its product into the cytoplasmic membrane. J Bacteriol. 1986 Apr;166(1):244–252. doi: 10.1128/jb.166.1.244-252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz B., Berg H. C. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J Bacteriol. 1991 Nov;173(21):7033–7037. doi: 10.1128/jb.173.21.7033-7037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T., Tanabe Y., Shigemoto R., Iwai M., Takumi T., Ohkubo H., Nakanishi S. Immunohistochemical study of a rat membrane protein which induces a selective potassium permeation: its localization in the apical membrane portion of epithelial cells. J Membr Biol. 1990 Jan;113(1):39–47. doi: 10.1007/BF01869604. [DOI] [PubMed] [Google Scholar]
- Sugiyama S., Cragoe E. J., Jr, Imae Y. Amiloride, a specific inhibitor for the Na+-driven flagellar motors of alkalophilic Bacillus. J Biol Chem. 1988 Jun 15;263(17):8215–8219. [PubMed] [Google Scholar]
- Sugrue R. J., Hay A. J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991 Feb;180(2):617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tobe T., Sasakawa C., Okada N., Honma Y., Yoshikawa M. vacB, a novel chromosomal gene required for expression of virulence genes on the large plasmid of Shigella flexneri. J Bacteriol. 1992 Oct;174(20):6359–6367. doi: 10.1128/jb.174.20.6359-6367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S. G., Armarego W. L., Shaw D. C., Lilley P. E., Dixon N. E., Poole R. K. Isolation and nucleotide sequence of the hmp gene that encodes a haemoglobin-like protein in Escherichia coli K-12. Mol Gen Genet. 1991 Apr;226(1-2):49–58. doi: 10.1007/BF00273586. [DOI] [PubMed] [Google Scholar]
- Wilson M. L., Macnab R. M. Co-overproduction and localization of the Escherichia coli motility proteins motA and motB. J Bacteriol. 1990 Jul;172(7):3932–3939. doi: 10.1128/jb.172.7.3932-3939.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. A., Smith J. M. Nucleotide sequence and analysis of the purA gene encoding adenylosuccinate synthetase of Escherichia coli K12. J Biol Chem. 1988 Dec 15;263(35):19147–19153. [PubMed] [Google Scholar]
- Zuberi A. R., Ying C. W., Parker H. M., Ordal G. W. Transposon Tn917lacZ mutagenesis of Bacillus subtilis: identification of two new loci required for motility and chemotaxis. J Bacteriol. 1990 Dec;172(12):6841–6848. doi: 10.1128/jb.172.12.6841-6848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]