Abstract
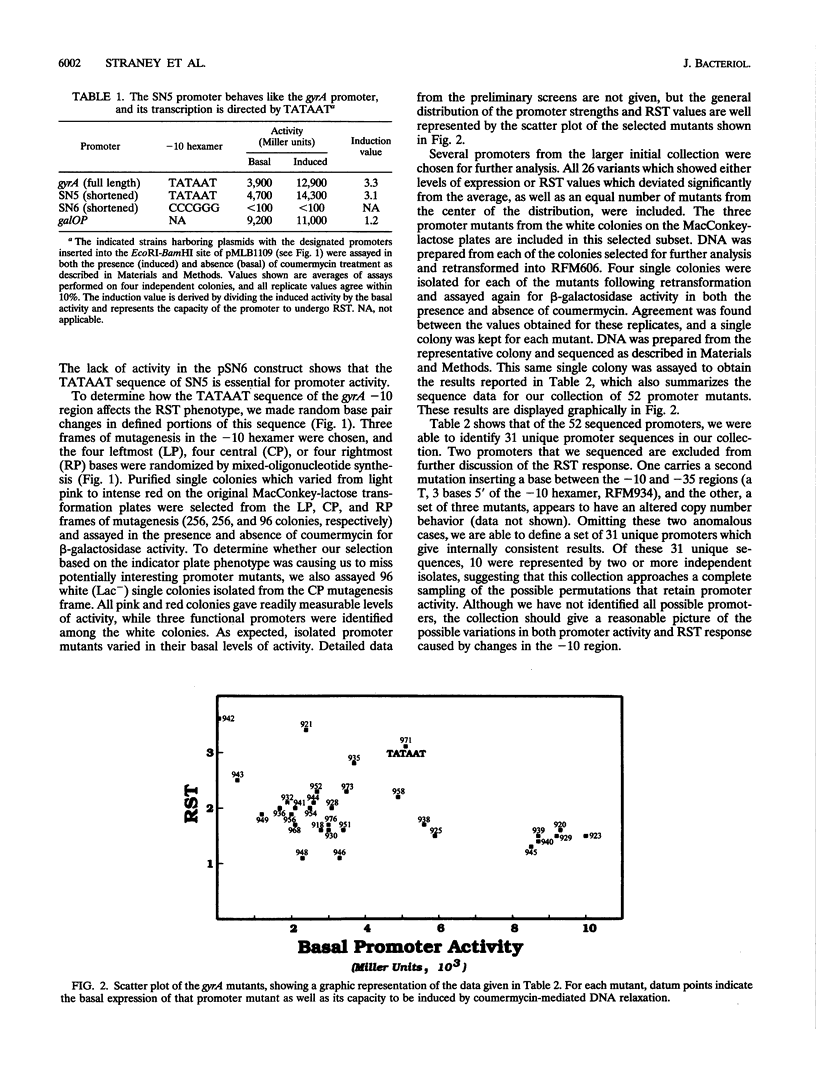
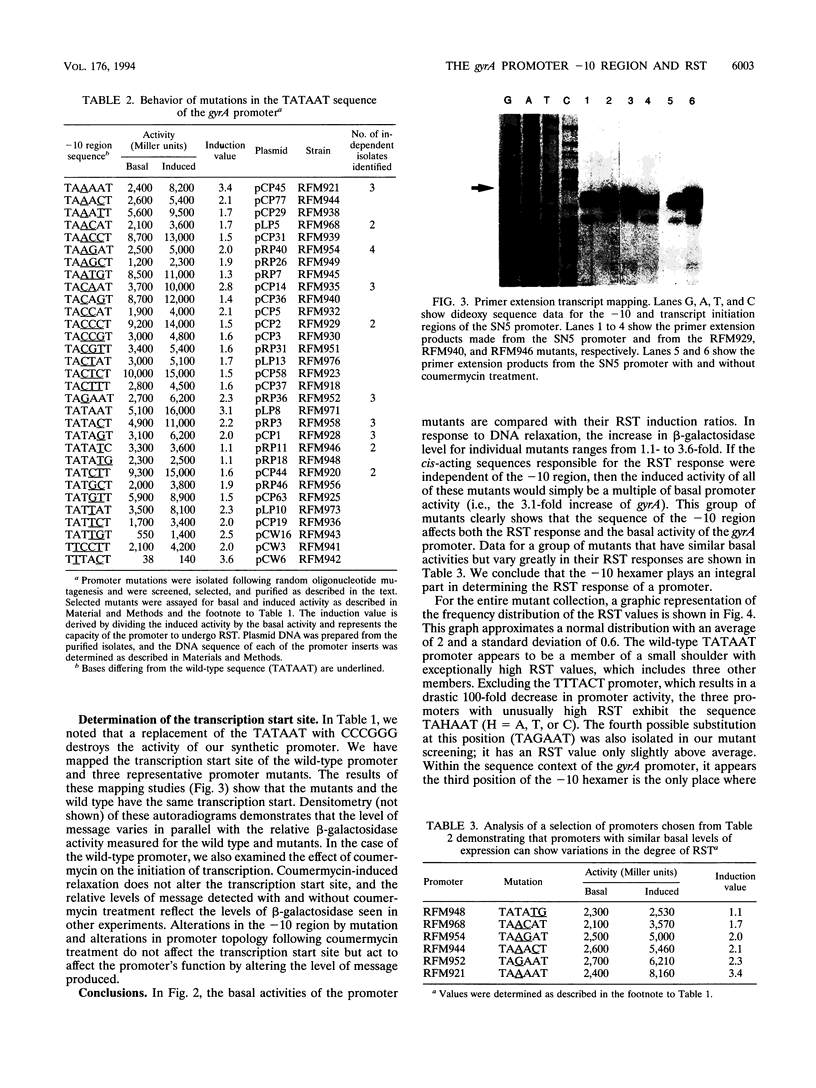
Transcription of the gyrA and gyrB genes, which encode the subunits of DNA gyrase, increases in response to DNA relaxation. Previous studies have shown that a small segment of DNA extending from the -10 consensus hexamer to the start of transcription encodes the sequence determinants for this response. In this study, we examined the role of the -10 region in relaxation-stimulated transcription (RST). A synthetic derivative of the gyrA promoter was designed to allow the modular replacement of the -10 region, and mixed-oligonucleotide mutagenesis was used to obtain a collection of promoter mutants. Most substitutions result in a reduction of the promoter's RST response, and some mutations abolish it altogether. We also note that a variety of promoter changes can increase basal expression twofold above that seen for the gyrA promoter, despite sequences changes (up to three bases) which diverge from the consensus TATAAT of the wild-type gyrA hexamer. The wild-type gyrA promoter, however, is the strongest promoter in this collection on a relaxed DNA template and appears to be repressed on a supercoiled template in vivo. Our results are consistent with a mechanism for RST that involves a step in transcription initiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Mizuuchi K., Menzel R., Gellert M. DNA sequence and transcription of the region upstream of the E. coli gyrB gene. Nucleic Acids Res. 1984 Aug 24;12(16):6389–6395. doi: 10.1093/nar/12.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. All three elements of the lac ps promoter mediate its transcriptional response to DNA supercoiling. J Mol Biol. 1987 May 5;195(1):89–97. doi: 10.1016/0022-2836(87)90329-9. [DOI] [PubMed] [Google Scholar]
- Botchan P., Wang J. C., Echols H. Effect of circularity and superhelicity on transcription from bacteriophagelambda DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3077–3081. doi: 10.1073/pnas.70.11.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms J. G., Dargouge O., Brahms S., Ohara Y., Vagner V. Activation and inhibition of transcription by supercoiling. J Mol Biol. 1985 Feb 20;181(4):455–465. doi: 10.1016/0022-2836(85)90419-x. [DOI] [PubMed] [Google Scholar]
- Carty M., Menzel R. Inhibition of DNA gyrase activity in an in vitro transcription-translation system stimulates gyrA expression in a DNA concentration dependent manner. Evidence for the involvement of factors which may be titrated. J Mol Biol. 1990 Jul 20;214(2):397–406. doi: 10.1016/0022-2836(90)90189-S. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Graña D., Gardella T., Susskind M. M. The effects of mutations in the ant promoter of phage P22 depend on context. Genetics. 1988 Oct;120(2):319–327. doi: 10.1093/genetics/120.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Hayashi M. Template activities of the phi X-174 replicative allomorphic deoxyribonucleic acids. Biochemistry. 1971 Nov;10(23):4212–4218. doi: 10.1021/bi00799a009. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Purification of ribonuclease H as a factor required for initiation of in vitro Co1E1 DNA replication. Nucleic Acids Res. 1982 Oct 11;10(19):5949–5965. doi: 10.1093/nar/10.19.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade J. M., deHaseth P. L. Bacteriophage lambda promoters pL and pR: sequence determinants of in vivo activity and of sensitivity to the DNA gyrase inhibitor, coumermycin. Gene. 1991 Jan 2;97(1):7–12. doi: 10.1016/0378-1119(91)90003-t. [DOI] [PubMed] [Google Scholar]
- Lisser S., Margalit H. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 1993 Apr 11;21(7):1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. Rate-limiting steps in RNA chain initiation. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5634–5638. doi: 10.1073/pnas.77.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. A microtiter plate-based system for the semiautomated growth and assay of bacterial cells for beta-galactosidase activity. Anal Biochem. 1989 Aug 15;181(1):40–50. doi: 10.1016/0003-2697(89)90391-6. [DOI] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Fusions of the Escherichia coli gyrA and gyrB control regions to the galactokinase gene are inducible by coumermycin treatment. J Bacteriol. 1987 Mar;169(3):1272–1278. doi: 10.1128/jb.169.3.1272-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Modulation of transcription by DNA supercoiling: a deletion analysis of the Escherichia coli gyrA and gyrB promoters. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4185–4189. doi: 10.1073/pnas.84.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Effects of supercoiling on transcription from bacteriophage PM2 deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3164–3169. doi: 10.1021/bi00712a025. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Initiation of transcription by Escherichia coli RNA polymerase from supercoiled and non-supercoiled bacteriophage PM2 DNA. J Mol Biol. 1975 Feb 5;91(4):477–487. doi: 10.1016/0022-2836(75)90274-0. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Siegele D. A., Hu J. C., Walter W. A., Gross C. A. Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989 Apr 20;206(4):591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- Waldburger C., Gardella T., Wong R., Susskind M. M. Changes in conserved region 2 of Escherichia coli sigma 70 affecting promoter recognition. J Mol Biol. 1990 Sep 20;215(2):267–276. doi: 10.1016/s0022-2836(05)80345-6. [DOI] [PubMed] [Google Scholar]
- Wood D. C., Lebowitz J. Effect of supercoiling on the abortive initiation kinetics of the RNA-I promoter of ColE1 plasmid DNA. J Biol Chem. 1984 Sep 25;259(18):11184–11187. [PubMed] [Google Scholar]