Abstract
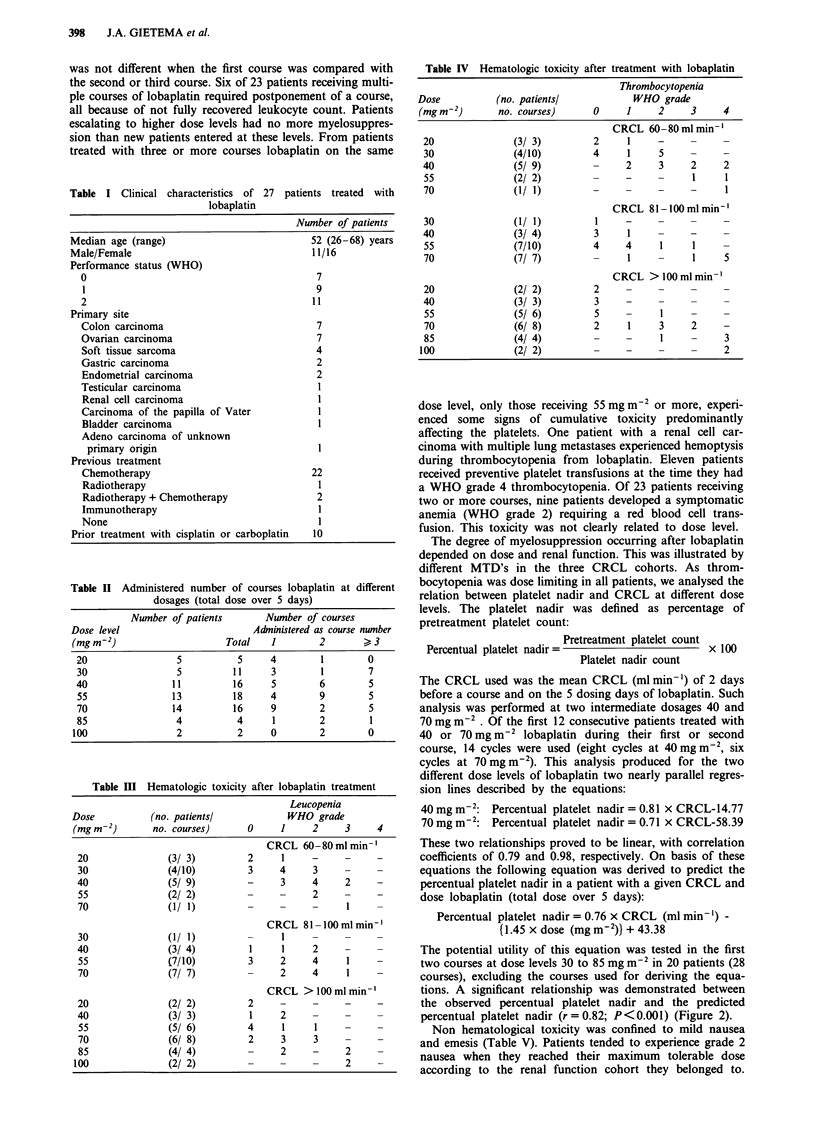
A phase I trial was conducted with lobaplatin (D-19466; 1,2-diamminomethyl-cyclobutane-platinum (II)-lactate) i.v. bolus daily for 5 days every 4 weeks. After entering five patients toxicity appeared to be related to renal function, therefore the individual dose (total dose 20-100 mg m-2 over 5 days) of lobaplatin was modified according to creatinine clearance (CRCL) and escalated in patients. Twenty-seven patients with refractory solid tumours received 72 courses. Thrombocytopenia was dose-limiting, its degree was related to dose and CRCL at time of drug administration. With a CRCL of 60-80 ml min-1 the maximum tolerated dose was 40 mg m-2, with a CRCL of 81-100 ml min-1 70 mg m-2, and with a CRCL > 100 ml min-1 it was 85 mg m-2. Platelet and leukocyte nadirs were observed around day 21. The percentual platelet nadir (percentage of day 1 platelet count) correlated with CRCL at different dose levels and could be described by 0.76 x CRCL (ml min-1) - (1.45 x dose (mg m-2) + 43.38. This equation tested in 20 patients (28 courses) produced a correlation between observed and predicted percentual platelet nadir (r = 0.82, P < 0.001). No renal function impairment occurred. Urinary excretion of platinum (by A.A.S) was estimated in six patients and revealed that 91.5% (s.e. +/- 7.9) of the platinum dose was excreted within 4 h. Responses (one PR, one CR) occurred in two patients with ovarian cancer (both pretreated with carboplatin and cisplatin). The recommended dose of lobaplatin i.v. bolus daily for 5 days for phase II studies depends on renal function, namely 30 mg m-2 at CRCL 60-80 ml min-1; 55 mg m-2 at CRCL 81-100 ml min-1; 70 mg m-2 at CRCL > 100 ml min-1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvert A. H., Newell D. R., Gumbrell L. A., O'Reilly S., Burnell M., Boxall F. E., Siddik Z. H., Judson I. R., Gore M. E., Wiltshaw E. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989 Nov;7(11):1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- Canetta R., Bragman K., Smaldone L., Rozencweig M. Carboplatin: current status and future prospects. Cancer Treat Rev. 1988 Jun;15 (Suppl B):17–32. doi: 10.1016/0305-7372(88)90031-x. [DOI] [PubMed] [Google Scholar]
- Egorin M. J., Van Echo D. A., Tipping S. J., Olman E. A., Whitacre M. Y., Thompson B. W., Aisner J. Pharmacokinetics and dosage reduction of cis-diammine(1,1-cyclobutanedicarboxylato)platinum in patients with impaired renal function. Cancer Res. 1984 Nov;44(11):5432–5438. [PubMed] [Google Scholar]
- Einhorn L. H., Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med. 1977 Sep;87(3):293–298. doi: 10.7326/0003-4819-87-3-293. [DOI] [PubMed] [Google Scholar]
- Gralla R. J., Cvitkovic E., Golbey R. B. cis-Dichlorodiammineplatinum(II) in non-small cell carcinoma of the lung. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1585–1588. [PubMed] [Google Scholar]
- Hansen H. H., Selawry O. S., Muggia F. M., Walker M. D. Clinical studies with 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (NSC 79037). Cancer Res. 1971 Mar;31(3):223–227. [PubMed] [Google Scholar]
- LeRoy A. F., Wehling M. L., Sponseller H. L., Friauf W. S., Solomon R. E., Dedrick R. L., Litterst C. L., Gram T. E., Guarino A. M., Becker D. A. Analysis of platinum in biological materials by flameless atomic absorption spectrophotometry. Biochem Med. 1977 Oct;18(2):184–191. doi: 10.1016/0006-2944(77)90089-8. [DOI] [PubMed] [Google Scholar]
- Mangioni C., Bolis G., Pecorelli S., Bragman K., Epis A., Favalli G., Gambino A., Landoni F., Presti M., Torri W. Randomized trial in advanced ovarian cancer comparing cisplatin and carboplatin. J Natl Cancer Inst. 1989 Oct 4;81(19):1464–1471. doi: 10.1093/jnci/81.19.1464. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969 Apr 26;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Rozencweig M., Nicaise C., Beer M., Crespeigne N., Van Rijmenant M., Lenaz L., Kenis Y. Phase I study of carboplatin given on a five-day intravenous schedule. J Clin Oncol. 1983 Oct;1(10):621–626. doi: 10.1200/JCO.1983.1.10.621. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Fukuda M., Morita M., Shinkai T., Eguchi K., Tamura T., Ohe Y., Yamada K., Kojima A., Nakagawa K. Prediction from creatinine clearance of thrombocytopenia and recommended dose in patients receiving (glycolato-O,O')-diammine platinum (II) (NSC 375101D). Jpn J Cancer Res. 1990 Feb;81(2):196–200. doi: 10.1111/j.1349-7006.1990.tb02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway M. S. Cis-diamminedichloroplatinum II in advanced urothelial cancer. J Urol. 1978 Dec;120(6):716–719. doi: 10.1016/s0022-5347(17)57339-5. [DOI] [PubMed] [Google Scholar]
- Van Echo D. A., Egorin M. J., Whitacre M. Y., Olman E. A., Aisner J. Phase I clinical and pharmacologic trial of carboplatin daily for 5 days. Cancer Treat Rep. 1984 Sep;68(9):1103–1114. [PubMed] [Google Scholar]
- Wiltshaw E., Kroner T. Phase II study of cis-dichlorodiammineplatinum(II) (NSC-119875) in advanced adenocarcinoma of the ovary. Cancer Treat Rep. 1976 Jan;60(1):55–60. [PubMed] [Google Scholar]



