Abstract
A panel of six 'wild type' and three VP-16 resistant small cell lung cancer (SCLC) cell lines is used to evaluate to what extent in vitro sensitivity testing using a clonogenic assay can contribute to combine cytotoxic drugs to regimens with improved efficacy against SCLC. The resistant lines include (a) H69/DAU4, which is classical multidrug resistant (MDR) with a P-glycoprotein efflux pump (b) NYH/VM, which exhibits an altered topoisomerase II (topo II) activity and (c) H69/VP, which is cross-resistant to vincristine, exhibits a reduced drug accumulation as H69/DAU4 but is without P-glycoprotein. 19 anticancer agents were compared in the panel. The MDR lines demonstrated, as expected, cross-resistance to all topo II drugs, but also different patterns of collateral sensitivity to BCNU, cisplatin, ara-C, hydroxyurea, and to the topo I inhibitor camptothecin. The complete panel of nine cell lines clearly demonstrated diverse sensitivity patterns to drugs with different modes of action. Correlation analysis showed high correlation coefficients (CC) among drug analogues (e.g. VP-16/VM-26 0.99, vincristine/vindesine 0.89), and between drugs with similar mechanisms of action (e.g. BCNU/Cisplatin 0.89, VP-16/Doxorubicin 0.92), whereas different drug classes demonstrated low or even negative CC (e.g. BCNU/VP-16 -0.21). When the CC of the 19 drug patterns to VP-16 were plotted against the CC to BCNU, clustering was observed between drugs acting on microtubules, on topo II, alkylating agents, and antimetabolites. In this plot, camptothecin and ara-C patterns were promising by virtue of their lack of cross-resistance to alkylating agents and topo II drugs. Thus, the differential cytotoxicity patterns on this panel of cells can (1) give information about drug mechanism of action, (2) enable the selection and combination of non-cross-resistant drugs, and (3) show where new drugs 'fit in' among established agents.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai R. L., Paull K. D., Herald C. L., Malspeis L., Pettit G. R., Hamel E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem. 1991 Aug 25;266(24):15882–15889. [PubMed] [Google Scholar]
- Bepler G., Jaques G., Neumann K., Aumüller G., Gropp C., Havemann K. Establishment, growth properties, and morphological characteristics of permanent human small cell lung cancer cell lines. J Cancer Res Clin Oncol. 1987;113(1):31–40. doi: 10.1007/BF00389964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen H. H., de Leij L., de Vries E. G., Mesander G., Mulder N. H., de Jong B., Buys C. H., Postmus P. E., Poppema S., Sluiter H. J. Characterization of three small cell lung cancer cell lines established from one patient during longitudinal follow-up. Cancer Res. 1988 Dec 1;48(23):6891–6899. [PubMed] [Google Scholar]
- Bork E., Ersbøll J., Dombernowsky P., Bergman B., Hansen M., Hansen H. H. Teniposide and etoposide in previously untreated small-cell lung cancer: a randomized study. J Clin Oncol. 1991 Sep;9(9):1627–1631. doi: 10.1200/JCO.1991.9.9.1627. [DOI] [PubMed] [Google Scholar]
- Campling B. G., Pym J., Baker H. M., Cole S. P., Lam Y. M. Chemosensitivity testing of small cell lung cancer using the MTT assay. Br J Cancer. 1991 Jan;63(1):75–83. doi: 10.1038/bjc.1991.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell B. M., Bozzino J. M., Corris P., Harris A. L. The multidrug resistant phenotype in clinical practice; evaluation of cross resistance to ifosfamide and mesna after VP16-213, doxorubicin and vincristine (VPAV) for small cell lung cancer. Eur J Cancer Clin Oncol. 1988 Feb;24(2):123–129. doi: 10.1016/0277-5379(88)90242-8. [DOI] [PubMed] [Google Scholar]
- Carmichael J., Mitchell J. B., DeGraff W. G., Gamson J., Gazdar A. F., Johnson B. E., Glatstein E., Minna J. D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br J Cancer. 1988 Jun;57(6):540–547. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Cole S. P., Chanda E. R., Dicke F. P., Gerlach J. H., Mirski S. E. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991 Jul 1;51(13):3345–3352. [PubMed] [Google Scholar]
- Cole S. P. The 1991 Merck Frosst Award. Multidrug resistance in small cell lung cancer. Can J Physiol Pharmacol. 1992 Mar;70(3):313–329. doi: 10.1139/y92-040. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Workman P., Twentyman P. R. Retention of activity by selected anthracyclines in a multidrug resistant human large cell lung carcinoma line without P-glycoprotein hyperexpression. Br J Cancer. 1991 Mar;63(3):351–357. doi: 10.1038/bjc.1991.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks M. K., Schmidt C. A., Cirtain M. C., Suttle D. P., Beck W. T. Altered catalytic activity of and DNA cleavage by DNA topoisomerase II from human leukemic cells selected for resistance to VM-26. Biochemistry. 1988 Nov 29;27(24):8861–8869. doi: 10.1021/bi00424a026. [DOI] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Ferguson P. J., Fisher M. H., Stephenson J., Li D. H., Zhou B. S., Cheng Y. C. Combined modalities of resistance in etoposide-resistant human KB cell lines. Cancer Res. 1988 Nov 1;48(21):5956–5964. [PubMed] [Google Scholar]
- Franco R., Kraft T., Miller T., Popp M., Martelo O. Storage of chemotherapy drugs for use in the human tumor stem cell assay. Int J Cell Cloning. 1984 Jan;2(1):2–8. doi: 10.1002/stem.5530020103. [DOI] [PubMed] [Google Scholar]
- Hansen H. H. Management of small-cell cancer of the lung. Lancet. 1992 Apr 4;339(8797):846–849. doi: 10.1016/0140-6736(92)90287-d. [DOI] [PubMed] [Google Scholar]
- Jensen P. B., Jensen P. S., Demant E. J., Friche E., Sørensen B. S., Sehested M., Wassermann K., Vindeløv L., Westergaard O., Hansen H. H. Antagonistic effect of aclarubicin on daunorubicin-induced cytotoxicity in human small cell lung cancer cells: relationship to DNA integrity and topoisomerase II. Cancer Res. 1991 Oct 1;51(19):5093–5099. [PubMed] [Google Scholar]
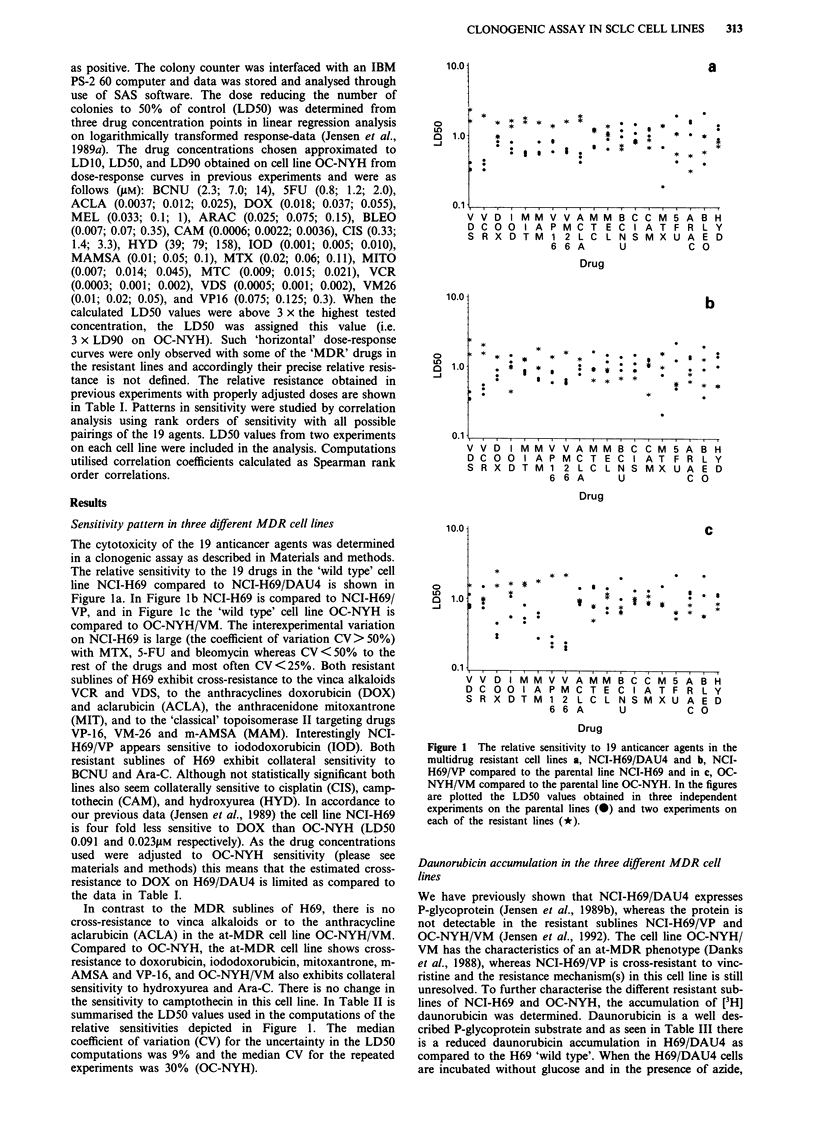
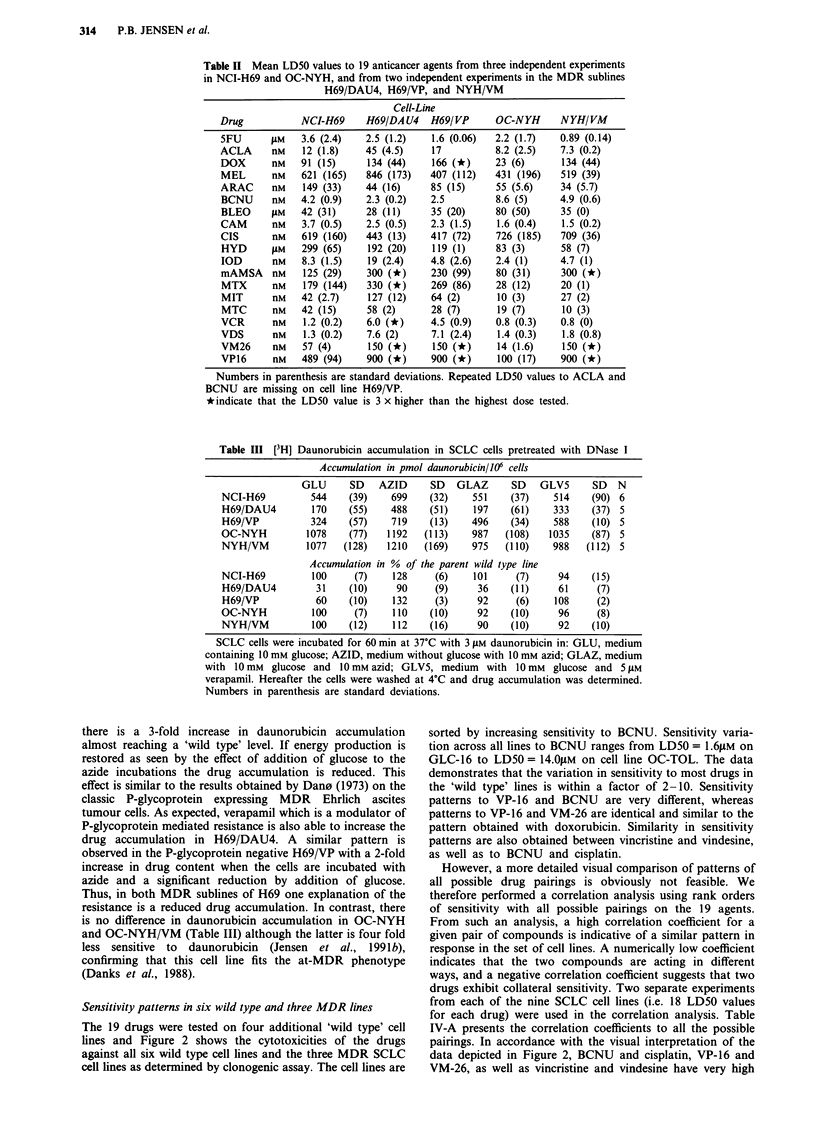
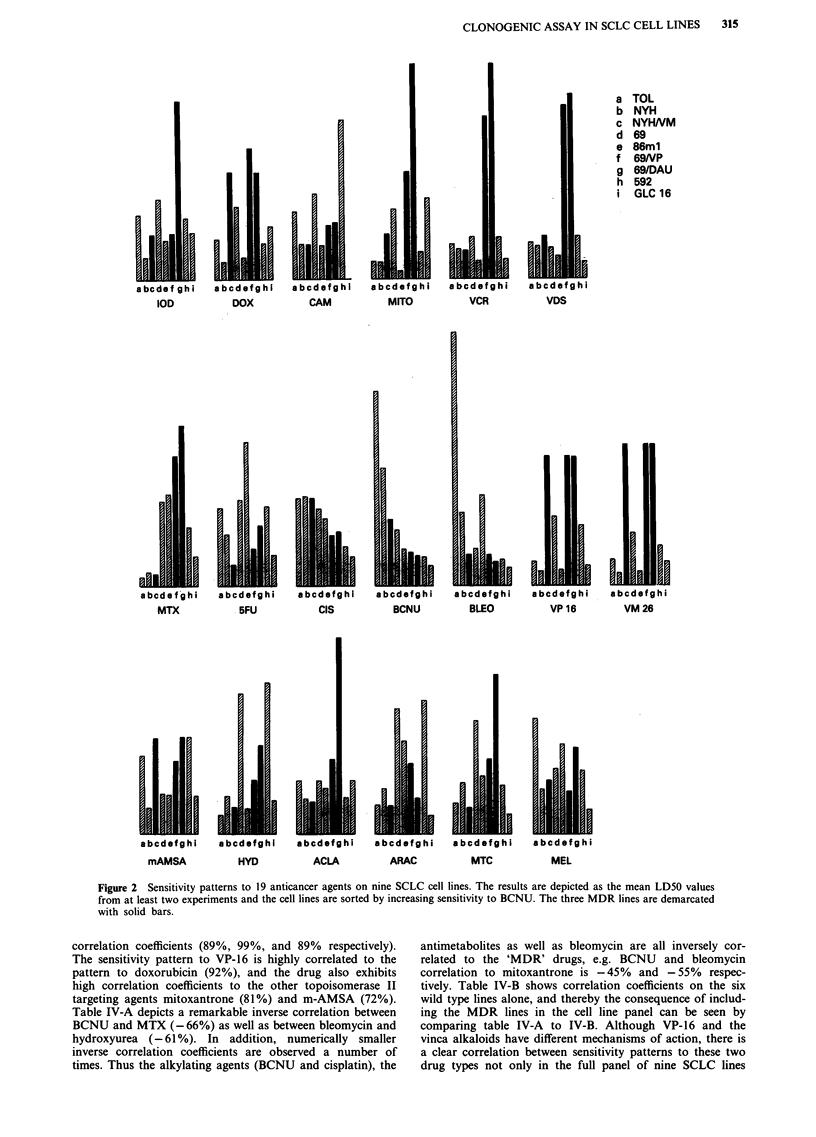
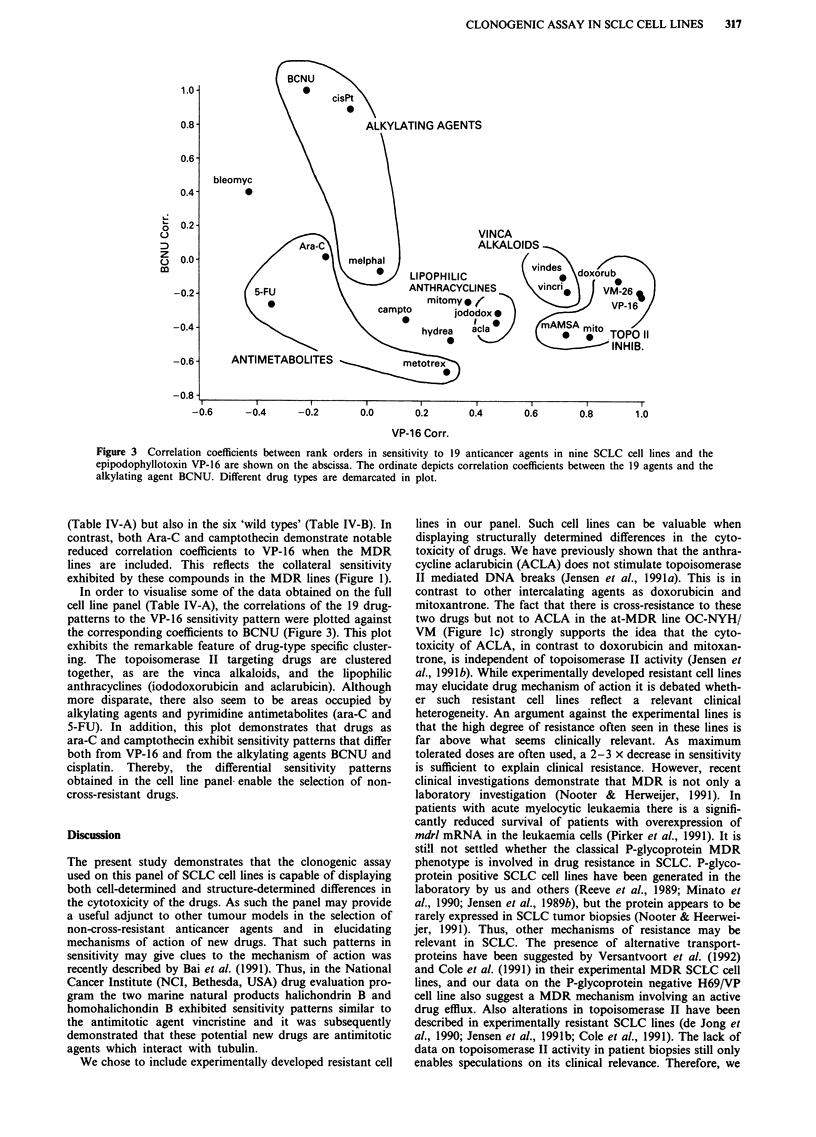
- Jensen P. B., Roed H., Sehested M., Demant E. J., Vindeløv L., Christensen I. J., Hansen H. H. Doxorubicin sensitivity pattern in a panel of small-cell lung-cancer cell lines: correlation to etoposide and vincristine sensitivity and inverse correlation to carmustine sensitivity. Cancer Chemother Pharmacol. 1992;31(1):46–52. doi: 10.1007/BF00695993. [DOI] [PubMed] [Google Scholar]
- Jensen P. B., Roed H., Vindeløv L., Christensen I. J., Hansen H. H. Reduced variation in the clonogenic assay obtained by standardization of the cell culture conditions prior to drug testing on human small cell lung cancer cell lines. Invest New Drugs. 1989 Nov;7(4):307–315. doi: 10.1007/BF00173760. [DOI] [PubMed] [Google Scholar]
- Jensen P. B., Vindeløv L., Roed H., Demant E. J., Sehested M., Skovsgaard T., Hansen H. H. In vitro evaluation of the potential of aclarubicin in the treatment of small cell carcinoma of the lung (SCCL). Br J Cancer. 1989 Dec;60(6):838–844. doi: 10.1038/bjc.1989.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen E., Bonven B. J., Andoh T., Ishii K., Okada K., Bolund L., Westergaard O. Characterization of a camptothecin-resistant human DNA topoisomerase I. J Biol Chem. 1988 Mar 15;263(8):3912–3916. [PubMed] [Google Scholar]
- Kristjansen P. E., Hirsch F. R. A review of the 5th World Conference on Lung Cancer held by the International Association for the Study of Lung Cancer. Eur Respir J. 1989 Mar;2(3):275–279. [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Long B. H., Wang L., Lorico A., Wang R. C., Brattain M. G., Casazza A. M. Mechanisms of resistance to etoposide and teniposide in acquired resistant human colon and lung carcinoma cell lines. Cancer Res. 1991 Oct 1;51(19):5275–5283. [PubMed] [Google Scholar]
- Mattern M. R., Hofmann G. A., McCabe F. L., Johnson R. K. Synergistic cell killing by ionizing radiation and topoisomerase I inhibitor topotecan (SK&F 104864). Cancer Res. 1991 Nov 1;51(21):5813–5816. [PubMed] [Google Scholar]
- Minato K., Kanzawa F., Nishio K., Nakagawa K., Fujiwara Y., Saijo N. Characterization of an etoposide-resistant human small-cell lung cancer cell line. Cancer Chemother Pharmacol. 1990;26(5):313–317. doi: 10.1007/BF02897284. [DOI] [PubMed] [Google Scholar]
- Nooter K., Herweijer H. Multidrug resistance (mdr) genes in human cancer. Br J Cancer. 1991 May;63(5):663–669. doi: 10.1038/bjc.1991.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker R., Wallner J., Geissler K., Linkesch W., Haas O. A., Bettelheim P., Hopfner M., Scherrer R., Valent P., Havelec L. MDR1 gene expression and treatment outcome in acute myeloid leukemia. J Natl Cancer Inst. 1991 May 15;83(10):708–712. doi: 10.1093/jnci/83.10.708. [DOI] [PubMed] [Google Scholar]
- Porter L. L., 3rd, Johnson D. H., Hainsworth J. D., Hande K. R., Greco F. A. Cisplatin and etoposide combination chemotherapy for refractory small cell carcinoma of the lung. Cancer Treat Rep. 1985 May;69(5):479–481. [PubMed] [Google Scholar]
- Reeve J. G., Rabbitts P. H., Twentyman P. R. Amplification and expression of mdr1 gene in a multidrug resistant variant of small cell lung cancer cell line NCI-H69. Br J Cancer. 1989 Sep;60(3):339–342. doi: 10.1038/bjc.1989.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roed H., Christensen I. B., Vindeløv L. L., Spang-Thomsen M., Hansen H. H. Inter-experiment variation and dependence on culture conditions in assaying the chemosensitivity of human small cell lung cancer cell lines. Eur J Cancer Clin Oncol. 1987 Feb;23(2):177–186. doi: 10.1016/0277-5379(87)90012-5. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. V., Shoemaker R. H., Paull K. D., Simon R. M., Tosini S., Skehan P., Scudiero D. A., Monks A., Boyd M. R. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990 Jul 4;82(13):1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- Sehested M., Friche E., Jensen P. B., Demant E. J. Relationship of VP-16 to the classical multidrug resistance phenotype. Cancer Res. 1992 May 15;52(10):2874–2879. [PubMed] [Google Scholar]
- Skovsgaard T. Mechanisms of resistance to daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Jun;38(6):1785–1791. [PubMed] [Google Scholar]
- Smit E. F., de Vries E. G., Timmer-Bosscha H., de Leij L. F., Oosterhuis J. W., Scheper R. J., Weening J. J., Postmus P. E., Mulder N. H. In vitro response of human small-cell lung-cancer cell lines to chemotherapeutic drugs; no correlation with clinical data. Int J Cancer. 1992 Apr 22;51(1):72–78. doi: 10.1002/ijc.2910510115. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Tsukahara S., Oh-hara T., Liu L. F., Tsuruo T. Elevated expression of DNA topoisomerase II in camptothecin-resistant human tumor cell lines. Cancer Res. 1990 Dec 15;50(24):7962–7965. [PubMed] [Google Scholar]
- Tsai C. M., Ihde D. C., Kadoyama C., Venzon D., Gazdar A. F. Correlation of in vitro drug sensitivity testing of long-term small cell lung cancer cell lines with response and survival. Eur J Cancer. 1990;26(11-12):1148–1152. doi: 10.1016/0277-5379(90)90274-w. [DOI] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Pinedo H. M., de Vries E. G., Feller N., Kuiper C. M., Lankelma J. Energy-dependent processes involved in reduced drug accumulation in multidrug-resistant human lung cancer cell lines without P-glycoprotein expression. Cancer Res. 1992 Jan 1;52(1):17–23. [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry. 1990;11(7):753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- Wu L., Smythe A. M., Stinson S. F., Mullendore L. A., Monks A., Scudiero D. A., Paull K. D., Koutsoukos A. D., Rubinstein L. V., Boyd M. R. Multidrug-resistant phenotype of disease-oriented panels of human tumor cell lines used for anticancer drug screening. Cancer Res. 1992 Jun 1;52(11):3029–3034. [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]
- de Leij L., Postmus P. E., Buys C. H., Elema J. D., Ramaekers F., Poppema S., Brouwer M., van der Veen A. Y., Mesander G., The T. H. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 1985 Dec;45(12 Pt 1):6024–6033. [PubMed] [Google Scholar]
- de Vries E. G., Meijer C., Timmer-Bosscha H., Berendsen H. H., de Leij L., Scheper R. J., Mulder N. H. Resistance mechanisms in three human small cell lung cancer cell lines established from one patient during clinical follow-up. Cancer Res. 1989 Aug 1;49(15):4175–4178. [PubMed] [Google Scholar]



