Abstract
Water permeability measured between the airspace and vasculature in intact sheep and mouse lungs is high. More than 95% of the internal surface area of the lung is lined by alveolar epithelial type I cells. The purpose of this study was to test whether osmotic water permeability (Pf) in type I alveolar epithelial cells is high enough to account for the high Pf of the intact lung. Pf measured between the airspace and vasculature in the perfused fluid-filled rat lung by the pleural surface fluorescence method was high (0.019 ± 0.004 cm/s at 12°C) and weakly temperature-dependent (activation energy 3.7 kcal/mol). To resolve the contributions of type I and type II alveolar epithelial cells to lung water permeability, Pf was measured by stopped-flow light scattering in suspensions of purified type I or type II cells obtained by immunoaffinity procedures. In response to a sudden change in external solution osmolality from 300 to 600 mOsm, the volume of type I cells decreased rapidly with a half-time (t1/2) of 60–80 ms at 10°C, giving a plasma membrane Pf of 0.06–0.08 cm/s. Pf in type I cells was independent of osmotic gradient size and was weakly temperature-dependent (activation energy 3.4 kcal/mol). In contrast, t1/2 for type II cells in suspension was much slower, ≈1 s; Pf for type II cells was 0.013 cm/s. Vesicles derived from type I cells also had a very high Pf of 0.06–0.08 cm/s at 10°C that was inhibited 95% by HgCl2. The Pf in type I cells is the highest measured for any mammalian cell membrane and would account for the high water permeability of the lung.
Keywords: water transport, aquaporin, light scattering, pulmonary edema, epithelium
Rapid water movement between the airspace and blood compartments of the lung is important in normal physiological processes such as the transition from an intrauterine to air environment and in pathological processes such as the formation and resolution of pulmonary edema. Water movement also may be important in maintaining lung water homeostasis. Between the air and the vasculature there are epithelial, interstitial, and endothelial compartments. The alveolar epithelium, which covers more than 99% of the internal surface area of the lungs (1), is comprised of a monolayer of two morphologically distinct types of cells, type I cells and type II cells. The very thin cytoplasmic extensions of type I cells cover 95–98% of the surface area of the lung (2). Type II cells, which cover the remaining 2–5% of the alveolar surface, are cuboidal cells best known for their ability to synthesize, secrete, and recycle components of pulmonary surfactant. The interstitial compartment varies considerably in thickness; at its thinnest, the alveolar epithelium is separated from capillary endothelium only by a fused basement membrane (3). Intercellular tight junctions between alveolar epithelial cells are thought to provide a tight barrier between the air and blood compartments of the lung (4). Based on these anatomic considerations, we postulated that alveolar epithelial type I cells might play an important role in water transport.
Recent data indicate that water moves rapidly between the airspace and capillaries in response to osmotic gradients. In an in situ perfused sheep lung model, osmotic water permeability (Pf) was estimated from the time course of changes in airspace fluid osmolality after instillation of hypertonic (900 mOsm) fluid into the airspaces (5). Airspace fluid osmolality equilibrated rapidly (half-time ≈40 s), giving a high Pf value of 0.02 cm/s. Pf was weakly temperature-dependent and reversibly inhibited by HgCl2, implicating a transcellular route for water movement through molecular water channels (aquaporins). Similar functional results were reported recently in mouse lung by using a pleural surface fluorescence method in which airspace osmolality was measured from the fluorescence of an aqueous-phase dye instilled into the airspace compartment (6). Three aquaporins have been localized in lung: AQP1 in capillary endothelia and some pneumocytes (5, 7), AQP4 in basolateral membranes of airway epithelium (8), and AQP5 in apical membranes of alveolar epithelium (9). A new water channel (AQP8) also is expressed in lung, but its cellular localization has not been determined (10). The importance of these individual aquaporins in normal lung function is not known. Humans lacking AQP1 appear to develop normally (11), as do transgenic knockout mice lacking AQP4 (12). However, transgenic mice lacking AQP1 have a severe urinary concentrating defect (39); lung function in these mice is currently under investigation.
The purpose of this study was to test the hypotheses that the water permeability in rat lung is very high and that this high water permeability is attributable to type I cells. Measurements of water permeability between the airspace and vasculature in rat lung by a pleural surface fluorescence method showed a high Pf with functional evidence for the involvement of molecular water channels. Although there have been previous descriptions of methods to isolate type I cells (13–15), there have been no previous reports of functional assays of type I cells. We have developed an improved method of isolating type I cells based on immunoselection with cell-specific mAbs and magnetic beads. Type I cells obtained by this method and vesicles prepared from these cells were used for the measurement of osmotic water permeability. Both type I cells and vesicles prepared from type I cells had an exceptionally high plasma membrane Pf (0.06–0.08 cm/s), higher than that reported to date in other mammalian cell plasma membranes. Quantitative comparison of water permeability in intact lung with the Pf in type I cell plasma membranes suggests that the type I cell is responsible for the high in vivo lung water permeability.
METHODS
Cell Isolation.
Type I cells. For each isolation, lungs from two male Sprague–Dawley 250-g rats (Charles River Breeding Laboratories) were perfused via the pulmonary artery with RPMI medium 1640 containing 25 mM Hepes (solution I) at 37°C. The lungs were lavaged via the trachea six times with Ca2+Mg2+-free PBS containing 5 mM each EDTA and EGTA at 37°C and then were instilled with 10 ml of solution II (solution I with 10% dextran; Sigma) containing elastase at 4.5 units/ml (grade II, Boehringer Mannheim). The enzyme-instilled lungs were incubated at 37°C for 10 min; an additional 30 ml of the elastase solution was instilled continuously via the trachea over 30 min. After this enzymatic digestion, the trachea and large airways were dissected and discarded. The lung tissue was minced to 1-cubic mm fragments in solution II containing 20% fetal bovine serum and 100 μg of DNase/ml (type IV, 2 mg/ml, Sigma). The lung fragments were gently agitated by end-over-end rotation for 4 min and filtered once through 150-μm nylon mesh and then twice through 20-μm mesh (Tetko, Elmsford, NY). The resultant cell suspension was centrifuged for 20 min at 250 × g over a discontinuous Percoll (Pharmacia) gradient consisting (bottom to top) of 10 ml each of: Percoll, density 1.080; solution II, Percoll, density 1.032. After centrifugation, the band formed at the 1.080/solution II interface was harvested, diluted to 50 ml with solution I at 4°C, and centrifuged for 10 min at 250 × g.
Immunoselection with magnetic beads was used first to remove macrophages and type II cells and then to select type I cells. The cells harvested from the Percoll gradient were resuspended and incubated with 20 μg of rat IgG/ml and a mouse mAb specific for RTII70, an apical plasma membrane protein of rat type II cells (16), at room temperature for 10 min. Cells were centrifuged and resuspended in solution III [PBS containing 0.5% BSA (Sigma) and 50 μg of DNase I/ml (Sigma)] and magnetic beads coated with secondary antibodies, goat anti-rat IgG, and goat anti-mouse IgG (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. Macrophages and type II cells were removed by magnetic selection. The remaining cells then were incubated with mAbs against RTI40, an integral membrane protein of type I cells (15, 17) [also called OTS-8 (18), SF-1 (19), T1α (20)] for 10 min. Cells were centrifuged, resuspended in solution III, and then incubated with rat anti-mouse IgG1 magnetic beads (Miltenyi) before magnetic selection. Cells were studied immediately after isolation.
Cell purity was determined by immunofluorescence of cytocentrifuged preparations by using mAbs specific for RTI40. Yields were 0.5–5 × 106 cells/rat. The percentage of type I cells ranged from 60% to 86%, with the major contaminants being type II cells and macrophages. Cells were examined before and after light scattering experiments; viability was greater than 95% by vital dye staining with fluorescein diacetate (Molecular Probes).
Alveolar macrophages and type II cells.
Alveolar macrophages were isolated from rat lungs by lavage with Ca2+Mg2+-free PBS containing 5 mM EDTA and 5 mM EGTA at 37°C. Yields were 5–10 × 106 macrophages/lung. Type II cells were isolated as previously described (21), with the following modification to remove type I cells (1–15%) present in some preparations of type II cells. mAb against RTI40 was added to the cells during the “panning” step, cells were centrifuged before adding goat anti-mouse IgG magnetic beads, and type I cells then were removed by negative magnetic selection (see above). The resultant cell preparations contained fewer than 0.2% type I cells by immunofluorescence and 90–95% type II cells by the modified Papanicolaou staining (22).
Preparation of Membrane Vesicles from Type I Cells.
Partially purified type I cell preparations containing 5 × 107 cells were homogenized by 35 strokes of a Dounce homogenizer at 4°C; vesicles were prepared by the method of Balch et al. (23). Bands were harvested from sucrose gradients, centrifuged, and resuspended in a solution of 5 mM Tris⋅HCl, pH 7.4, 50 mM sucrose and equilibrated at 4°C for 20 hr. Average vesicle volume was determined by quasi-elastic light scattering and by measurement of vesicle diameters using transmission electron microscopy. Protein was measured by the bicinchoninic acid method (Pierce, Rockford, IL). We measured RTI40 (15) and RTII70 (24) as biochemical markers for plasma membrane of type I and type II cells, respectively, as described previously (17). Alkaline phosphatase (5′-nucleotidase) was not used as a plasma membrane marker because type I cells (in contrast to type II cells) contain very little alkaline phosphatase (25).
Transmission Electron Microscopy.
Cells were fixed for 2 hr in 1% freshly prepared paraformaldehyde with or without 2% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4) and then postfixed overnight in 1.5% osmium tetroxide in veronal acetate buffer (pH 7.4). Tissue blocks were subsequently stained in sodium maleate-buffered uranyl acetate and dehydrated quickly in cold acetone and propylene oxide before embedding in LX 112 resin (Ladd Research Industries, Burlington, VT). Vesicles were fixed in 1% osmium tetroxide, 2% glutaraldehyde for 24 hr, then further fixed in 2% aqueous uranyl acetate; tissue blocks were treated as above but without propylene oxide. Thin sections were stained with 5% aqueous uranyl acetate and 0.8% lead citrate and examined in a Zeiss 10 transmission electron microscope.
Stopped-Flow Light Scattering Measurements.
Osmotic water permeability (Pf) was measured in suspensions of freshly isolated type I and type II alveolar epithelial cells by the stopped-flow light scattering method (26). Experiments were carried out on an Sf-51 stopped-flow apparatus (Hi-Tech, Wiltshire, U.K.) having solution mixing time of under 1 ms and a dead time of 1–2 ms. The apparatus was temperature-regulated. Equal 0.05-ml volumes of the cell suspension (1–5 × 106 cells/ml of solution I containing 10% dextran) were mixed with solution I containing sucrose to produce specified osmotic gradients. The time course of cell volume was measured by monitoring 90° scattered light intensity at a wavelength of 520 nm. The maximum rate of data acquisition and electronic bandwidth were 1 MHz. Pf was computed from the light scattering time course and cell surface-to-volume ratio as described previously (26). Pf also was measured in fractionated membrane vesicles from type I cells. Vesicles (8–40 μg protein/ml) were subjected to a 50 mM inwardly directed sucrose gradient in the stopped-flow apparatus.
Water Permeability in Perfused Lungs.
Sprague–Dawley rats (200 g) were euthanized with intraperitoneal pentobarbital (150 mg/kg). The trachea was immediately cannulated with polyethylene tubing, and the pulmonary artery was cannulated with PE-200 tubing. The heart and lungs were moved enbloc to a Lucite perfusion chamber for observation by epifluorescence microscopy as described previously (6). The pulmonary artery was gravity-perfused at constant pressure (15–20 cm H2O) and specified temperature. The airspace was filled with 2.5–3.0 ml of Hepes-buffered Ringer’s solution containing fluorescein isothiocyanate-dextran (70 kDa, 0.5 mg/ml) and the fluorescence intensity from a 3- to 5-mm diameter spot on the lung pleural surface was monitored.
An airspace-to-perfusate osmotic gradient was generated by switching the perfusate to Hepes-buffered Ringer’s solution with added sucrose (hyperosmolar). The time course of lung surface fluorescence was monitored continuously for studies performed for 30–60 min. Pf was computed from the relation: Pf = [d(F/Fo)/dt]t=0/[(S/Vo)vwΔC], where Pf (cm/s) is the osmotic water permeability coefficient, [d(F/Fo)/dt]t=0 is the initial slope of the relative fluorescence vs. time data, S (cm2) is surface area, vw (18 cm3/mol) is the partial molar volume of water, and ΔC is the difference in osmolality between perfusate and airspace fluids.
RESULTS
Type I Cell Preparations.
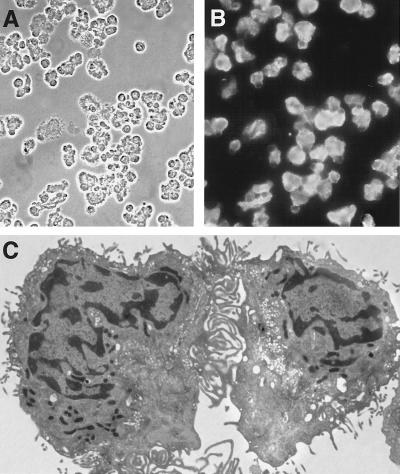
The percentage of type I cells in our cell preparations was assessed by indirect immunofluorescence with a mAb specific for RTI40, an integral apical membrane protein (15) specific within the lung for type I cells. Phase-contrast (Fig. 1A) and immunofluorescence (Fig. 1B) images of cytocentrifuged preparations of isolated cells demonstrated purities of between 60% and 86% in these studies. Electron microscopic images (Fig. 1C) of the cell preparations show morphologically complex cells with very thin cytoplasmic extensions, similar to those described previously for isolated type I cells (13, 14). More than 95% of the cells were viable as judged by uptake of the vital dye fluorescein diacetate (data not shown).
Figure 1.
Purity and appearance of type I cells. Cytocentrifuged cell preparation with (A) phase contrast and (B) immunofluorescence with a mAb specific for type I cells (C). Electron micrographs of isolated type I cells showing very thin cytoplasmic extensions typical of this cell type. Magnifications: A, ×280; B, ×300; C, ×4,150.
Osmotic Water Permeability of Isolated Alveolar Cells.
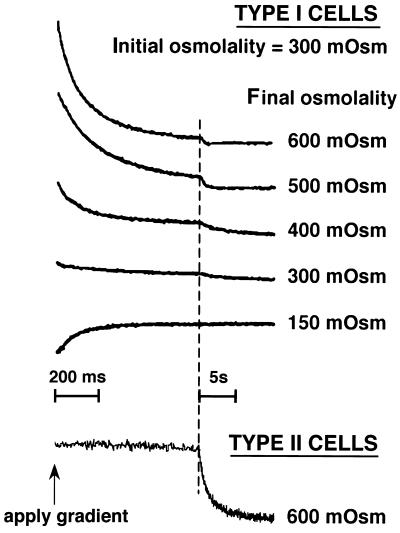
Stopped-flow measurements were performed in which cells, initially suspended in an isosmolar (300 mOsm) buffer, were subjected to different osmotic gradients. Fig. 2 (top curves) shows light scattering data for suspensions of type I cells. In each case, there was a very rapid (t1/2 approx. 60–80 ms) monophasic change in light scattering representing cell shrinking (for hyperosmotic gradients) or swelling (for the hyposmotic gradient). There was little signal change in the absence of an osmotic gradient. For the studies of cell shrinking, the signal amplitude increased approximately linearly with relative cell volume. The amplitude was smaller for the experiments in which cells were subjected to hyposmotic gradients; from measurements in other cell types, this is probably because of physical restrictions to swelling. Using a surface/volume ratio of 2.61 μm−1 based on morphometric measurements (2), Pf values for type I cells were 0.06–0.08 cm/s (four experiments, each with a different preparation of type I cells). Pf was independent of osmotic gradient size and direction.
Figure 2.
Stopped-flow light scattering measurement of osmotic water permeability in alveolar epithelial cells isolated from lung. Measurements were made at a 300-mOsm initial osmolality at 10°C as described in Methods. Representative data from one of three experiments are shown for purified type I cell preparations (top curves) and purified type II cells (bottom curve) with indicated final osmolalities. The same time scale was used for type I and type II cells to show the much slower water transport in type II cells.
The slower phase of signal change in these studies might represent signals from contaminating type II cells and/or alveolar macrophages. Fig. 2 (bottom curve) shows that exposure of purified type II cells to an osmotic gradient produces a slow volume change (t1/2 ≈ 1 s). Pf calculated from the stopped-flow light scattering data with type II cells was 0.013 cm/s, which is in good agreement with a previous measurement in isolated type II cells (5). Similar measurements with purified macrophages produced very small signals with relatively slow volume changes.
The parameters that have been used to implicate the involvement of molecular water channels are a high Pf of >0.01 cm/s, a low Arrhenius activation energy of <5 kcal/mol, inhibition of water transport by mercurials, and a ratio of osmotic-to-diffusional water permeability coefficients (Pf/Pd) of >1 (27, 28). It is not practical to measure Pd in type I cells because of their very high water permeability (labeled water equilibrium times would be <1 ms). Effects of mercurials on Pf could not be measured in intact type I cells because of marked HgCl2 toxicity. In 5 min, the viability of type I cells treated with 0.2 mM HgCl2 decreased to less than 50%; viability of control cells without HgCl2 remained 100%. We therefore measured mercurial sensitivity on vesicles prepared from cells (see below). The Arrhenius activation energy was determined from the temperature-dependence of Pf. Fig. 3 contains original light scattering data showing an increased rate of cell shrinkage with increasing temperature. An Arrhenius plot of ln Pf vs. reciprocal temperature gave an activation energy of 3.4 kcal/mol. This value suggests the involvement of molecular water channels.
Figure 3.
Temperature dependence of osmotic water permeability in type I cells. Light scattering data for a suspension of type I cells subjected to a 300-mOsm inwardly directly osmotic gradient at indicated temperatures. An Arrhenius plot of ln (Pf) vs. reciprocal absolute temperature (not shown) gave an activation energy (Ea) of 3.4 kcal/mol (r = 0.985 for linear fit).
Osmotic Water Permeability of Membrane Vesicles Prepared from Type I Cells.
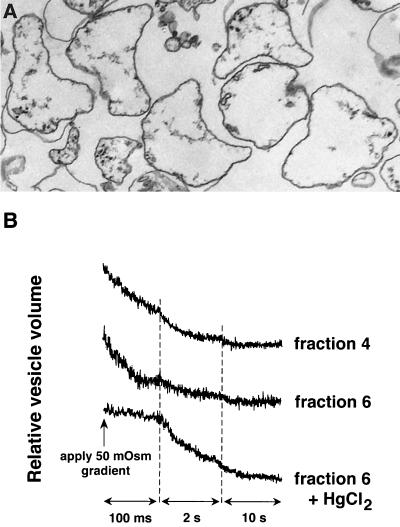
The very high Pf measured in isolated type I cells was computed by using published values for cell surface area and volume and assumes that water flow is not limited by unstirred layers. To determine Pf in membranes from type I cells in a manner that does not rely on assumptions about cell geometry and that is not affected by unstirred layers, we measured osmotic water permeability in vesicles. Light microsomes from homogenized type I cells were fractionated by sucrose density gradient centrifugation. Fig. 4B shows stopped-flow light scattering data for two gradient fractions: fractions 4 and 6. In fraction 6, there was a significant fast component of signal decrease (76% of total signal) with an exponential time constant of 68 ms, yielding a Pf value of 0.08 cm/s at 10°C. Pf was inhibited by 95% by 0.3 mM HgCl2 (Fig. 4B, bottom). Vesicles with high Pf also were seen in fraction 4, along with a vesicle population with a much lower Pf. The vesicles in both fractions were of similar sizes (≈430 nm diameter) by quasi-elastic light scattering and electron microscopy (fraction 6 shown in Fig. 4A). Both fractions 4 and 6 contained similar amounts of RTI40, a plasma membrane marker for type I cells; fraction 4 contained four times as much RTII70, a marker specific for plasma membranes of rat type II cells. Therefore, the slower component of light scattering in fraction 4 may be because of a greater content of membrane derived from type II cells. Although it was not possible to isolate apical and basolateral membrane vesicles individually from type I cells, these results indicate that a “mixed” membrane vesicle fraction has very high water permeability, consistent with the results obtained in intact type I cells.
Figure 4.
Osmotic water permeability in membrane vesicles isolated from type I cells. (A) Transmission electron micrograph of isolated vesicles from fraction 6. Magnification: ×33,000. (B) Stopped-flow light scattering measurement of osmotic water permeability of vesicle fractions 4 and 6. Vesicle suspensions were subjected to 50-mOsm inwardly directed osmotic gradients at 10°C. Both fractions contained a rapid component; fraction 4 contained in addition a slower component (see text for details). Treatment of fraction 6 treated with 0.3 mM HgCl2 inhibited Pf by 95%.
Osmotic Water Permeability in Intact Rat Lung.
A surface fluorescence method was used to measure osmotic water permeability of the alveolar epithelial barrier in intact lung. The rat lung was perfused ex vivo via the pulmonary artery as described previously (6), and the airspace was filled with 300 mOsm of saline containing fluorescein isothiocyanate (FITC)-dextran. In response to an increase in perfusate osmolality, water moves into the airspace and intra-alveolar FITC concentration increases, resulting in an increase in pleural surface fluorescence. Fig. 5A shows representative pleural surface fluorescence tracings obtained at 12°C, 23°C, and 37°C. Surface fluorescence changed promptly in response to changes in perfusate osmolality. Using an alveolar surface-to-volume ratio of 1,000 cm−1 [average alveolar diameter 59 μm (29)], a Pf of 0.019 ± 0.004 cm/s (12°C) was computed. Fig. 5B shows an Arrhenius plot for the temperature dependence of Pf, giving an activation energy of 3.7 kcal/mol.
Figure 5.
Osmotic water permeability between airspace and capillary compartments in rat lung. (A) Pleural surface fluorescence was recorded in response to indicated changes in perfusate osmolality at indicated temperatures. The airspace was filled with a 290-mOsm solution containing 0.5 mg/ml fluorescein isothiocyanate-dextran. (B) Arrhenius plot from which an activation energy of 3.7 kcal/mol was calculated.
DISCUSSION
Recent data indicates that water moves rapidly between the airspace and vasculature in response to osmotic gradients. The purpose of the current study was to determine whether type I cells, which cover more than 95% of the internal surface area of the lung, have a sufficiently high osmotic water permeability to be the major pathway for water permeability in the lung. Previous descriptions of methods to isolate type I cells (13–15) have demonstrated the morphologic characteristics of the isolated cells, but have not provided information about functions of type I cells. We used a combination of density gradient centrifugation and immunoselection with magnetic particles to isolate type I cells with 60% to 86% purity (based on cell number; the percentage of type I cell/total cell membrane would be higher) and >95% viability. When the isolated type I cells were subjected to osmotic gradients, there was a very rapid change in scattered light intensity, indicating a rapid change in cell volume. The calculated osmotic water permeability coefficient (Pf) of isolated type I cells was 0.06–0.08 cm/s, which represents the highest Pf measured in any mammalian cell. Calculation of Pf from light scattering data with isolated type I cells is complicated by uncertainties regarding the surface area/volume ratio of type I cells and by possible effects of unstirred layers. To obviate these problems, we measured water permeability in vesicles isolated from type I cell homogenates. Pf was very high, 0.08 cm/s, similar to that measured in intact type I cells. By analogy to results obtained for the renal epithelium of the descending limb of Henle, the data here suggest that a major function of type I cells may be the maintenance of a water permselective physical barrier.
The high water permeability between the airspace and vascular compartments in intact rat lung suggests the involvement of molecular water channels (aquaporins) in water transport. In rat airways and lung, AQP1 has been localized primarily to the alveolar endothelium (5, 7), AQP3 to trachea (8), AQP4 to tracheal and airway epithelia (8), and AQP5 to alveolar epithelium (9) (for review, see ref. 30). AQP5 is a mercurial-inhibitable water channel (31) that is expressed at the apical plasma membrane of type I cells (9). Pf in vesicles prepared from isolated type I cells was sensitive to mercurials; treatment with 0.3 mM HgCl2 inhibited Pf by approximately 95%. It is not known whether AQP5 is the only water channel of the type I cell apical membrane. Based on a recent estimate of the AQP5 single-channel water permeability of 5 × 10−14 cm3/s (32), an AQP5 density of ≈14,000 μm−2 would be required to account for the high type I cell Pf. Assuming that the plasma membrane is 50% protein, this high density suggests that AQP5 comprises ≈20% of apical membrane protein in type I cells. To date, no water channel has been identified on basolateral membranes of alveolar epithelium.
The osmotic water permeability of suspended type I cells measured here represents the composite water permeabilities of the apical and basolateral membranes weighted by their respective area fractions. The Pf of 0.06–0.08 at 10°C (0.09 cm/s at 23°C) is substantially higher than that of other “water permeable” mammalian cell membranes such as erythrocytes (0.02 cm/s) and cell plasma membranes of the renal proximal tubule (0.01–0.04 cm/s) (33) and the thin descending limb of Henle (0.06 cm/s). Pf measured in vesicles prepared from type I cells is also higher than that in vesicles prepared from a variety of mammalian cells and organs. Comparable very high Pf values have been reported only in endosomes from amphibian urinary bladder that contain the vasopressin-inducible water channel (0.1 cm/s; ref. 34). If apical and basolateral resistances to water flow are equal, the Pf of 0.07 cm/s for suspended type I cells predicts a Pf of ≈0.035 cm/s at 10°C, assuming no other resistances or parallel pathways for water movement. This Pf value is greater than that of 0.019 cm/s measured in intact rat lung, a result compatible with the hypothesis that type I cells provide a major route for water movement between the airspace and vascular compartments. The greater Pf predicted from the data on type I cells may be related to interstitial and endothelial barriers in intact lung, to the incomplete coverage of the alveolar surface with type I cells, and/or to unequal apical vs. basolateral membrane water permeabilities. In addition, the quantitative comparison of Pf values must be made with caution because of uncertainties in the surface-to-volume ratios of isolated type I cells and assumptions that cells and vesicles behave as perfect osmometers.
In summary, these results support the hypothesis that the type I cells play an important role in the high water permeability between the airspace and vasculature of the lung. The high water permeability of type I cells, together with previous studies of water channel expression and function in the lung, suggest an important role for aquaporin-type water channels in lung physiology. Water channels are expressed strongly in airway and alveolar epithelial and endothelial plasma membranes (5–9), water permeability is high across alveolar (5, 6) and airway (35) epithelia, and lung water channel expression (36, 37) and function (38) are developmentally regulated. Studies of lung functions in transgenic mice lacking specific water channels may help to define the precise roles of aquaporins in pulmonary physiology in normal and diseased states.
Acknowledgments
This work was supported by Grants HL57426, HL24075, DK35124, HL51854, and HL42368 from the National Institutes of Health, and Grant R613 from the National Cystic Fibrosis Foundation. E.P.C. was supported by a fellowship from the American Lung Association.
ABBREVIATION
- Pf
osmotic water permeability
References
- 1.Mercer R R, Russell M L, Roggli V L, Crapo J D. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 2.Stone K C, Mercer R R, Gehr P, Stockstill B, Crapo J D. Am J Respir Cell Mol Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 3.Weibel E W. Respir Physiol. 1970/1971;11:54–75. doi: 10.1016/0034-5687(70)90102-7. [DOI] [PubMed] [Google Scholar]
- 4.Schneeberger E E. Fed Proc. 1978;37:2471–2478. [PubMed] [Google Scholar]
- 5.Folkesson H G, Matthay M A, Hasegawa H, Kheradmand F, Verkman A S. Proc Natl Acad Sci USA. 1994;91:4970–4974. doi: 10.1073/pnas.91.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter E P, Matthay M A, Farinas J, Verkman A S. J Gen Physiol. 1996;108:133–142. doi: 10.1085/jgp.108.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondy C E, Chin E, Smith B L, Preston G M, Agre P. Proc Natl Acad Sci USA. 1993;90:4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frigeri A, Gropper M, Turck C W, Verkman A S. Proc Natl Acad Sci USA. 1995;92:4238–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen S, King L S, Christensen B M, Agre P. Am J Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 10.Ma T, Yang B, Verkman A S. Biochim Biophys Res Comm. 1997;240:324–328. doi: 10.1006/bbrc.1997.7664. [DOI] [PubMed] [Google Scholar]
- 11.Preston G M, Smith B L, Zeidel M L, Moulds J J, Agre P. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- 12.Ma T, Yang B, Gillespie A, Carlson E J, Epstein C J, Verkman A S. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller N K, Karnovsky M J. Am J Pathol. 1986;124:448–456. [PMC free article] [PubMed] [Google Scholar]
- 14.Picciano P, Rosenbaum R M. Am J Pathol. 1978;90:99–122. [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs L G, Williams M C, Gonzalez R. Biochim Biophys Acta. 1988;970:146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- 16.Dobbs L G, Pian M S, Maglio M, Dumars S, Allen L. Am J Physiol. 1997;273:L347–L354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 17.McElroy M C, Pittet J F, Hashimoto S, Allen L, Wiener-Kronish J P, Dobbs L G. Am J Physiol. 1995;268:L181–L186. doi: 10.1152/ajplung.1995.268.2.L181. [DOI] [PubMed] [Google Scholar]
- 18.Rishi A K, Joyce-Brady M, Fisher J, Dobbs L G, Floros J, VanderSpek J, Brody J S, Williams M C. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 19.Brody J S, Williams M C. Am Rev Physiol. 1992;54:351–371. doi: 10.1146/annurev.ph.54.030192.002031. [DOI] [PubMed] [Google Scholar]
- 20.Williams M C, Cao Y X, Hinds A, Rishi A, Wetterwald A. Am J Respir Cell Mol Biol. 1996;14:577–585. doi: 10.1165/ajrcmb.14.6.8652186. [DOI] [PubMed] [Google Scholar]
- 21.Dobbs L G, Gonzalez R, Williams M C. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 22.Dobbs L G. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 23.Balch W E, Dunphy W G, Braell W A, Rothman J E. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 24.Dobbs L G, Pian M, Dumars S, Maglio M, Allen L. Am J Physiol. 1997;273:L347–L354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 25.Edelson J D, Shannon J M, Mason R J. Am Rev Respir Dis. 1988;138:1268–1275. doi: 10.1164/ajrccm/138.5.1268. [DOI] [PubMed] [Google Scholar]
- 26.Van Hoek A N, Verkman A S. J Biol Chem. 1992;267:18267–18269. [PubMed] [Google Scholar]
- 27.Finkelstein A. Water Movement Through Lipid Bilayers, Pores, and Plasma Membranes: Theory and Reality. New York: Wiley; 1987. [Google Scholar]
- 28.Verkman A S, Van Hoek A N, Ma T, Frigeri A, Skach W R, Mitra A, Tamarappoo B K, Farinas J. Am J Physiol. 1996;270:C12–C30. doi: 10.1152/ajpcell.1996.270.1.C12. [DOI] [PubMed] [Google Scholar]
- 29.Crosfill M L, Widdicombe J G. J Physiol. 1961;158:1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthay M A, Folkesson H, Verkman A S. Am J Physiol. 1996;270:L487–L503. doi: 10.1152/ajplung.1996.270.4.L487. [DOI] [PubMed] [Google Scholar]
- 31.Raina S, Preston G M, Guggino W B, Agre P. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Verkman A S. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 33.Meyer M M, Verkman A S. J Membr Biol. 1987;96:107–119. doi: 10.1007/BF01869237. [DOI] [PubMed] [Google Scholar]
- 34.Shi L B, Brown D, Verkman A S. J Gen Physiol. 1990;95:941–960. doi: 10.1085/jgp.95.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folkesson H, Matthay M, Frigeri A, Verkman A S. J Clin Invest. 1996;97:664–671. doi: 10.1172/JCI118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King L S, Nielsen S, Agre P. J Clin Invest. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umenishi F, Carter E P, Yang B, Oliver B, Matthay M A, Verkman A S. Am J Respir Cell Mol Biol. 1996;15:673–679. doi: 10.1165/ajrcmb.15.5.8918374. [DOI] [PubMed] [Google Scholar]
- 38.Carter E P, Umenishi F, Matthay M A, Verkman A S. J Clin Invest. 1997;100:1071–1078. doi: 10.1172/JCI119617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma T, Yang B, Gillespie A, Carlson E J, Epstein C J, Verkman A S. J Biol Chem. 1997;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]