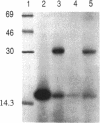
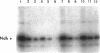
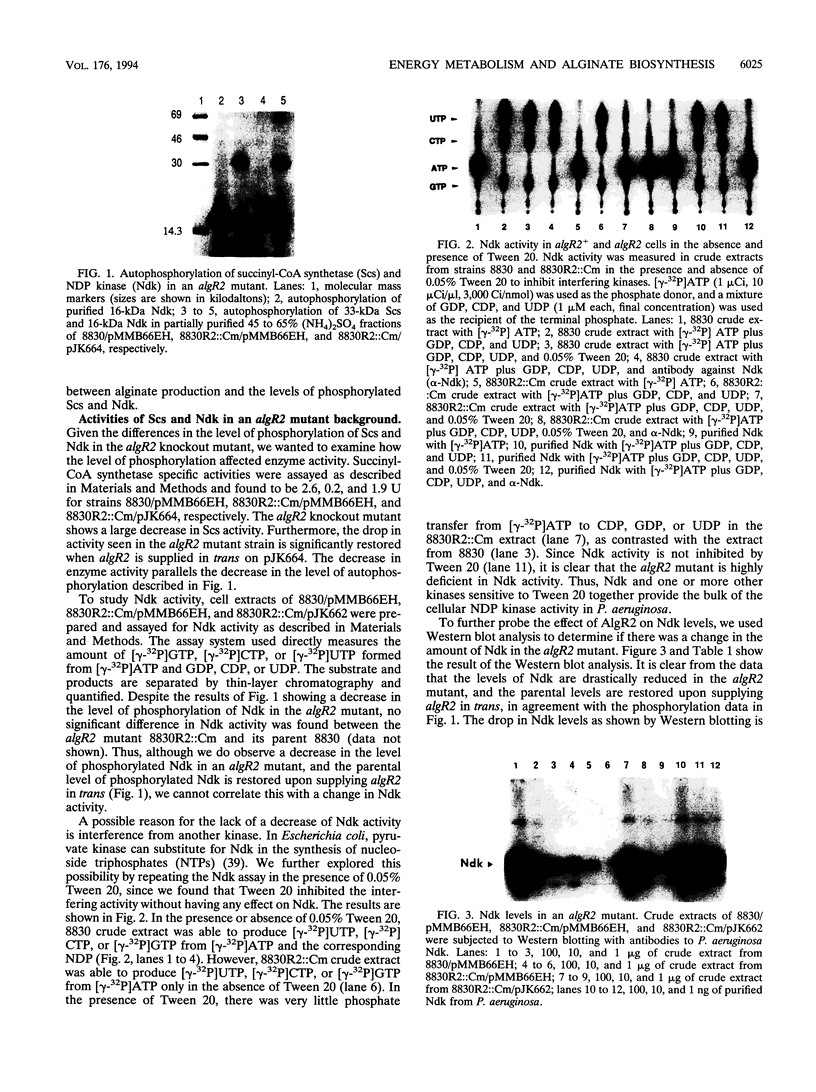
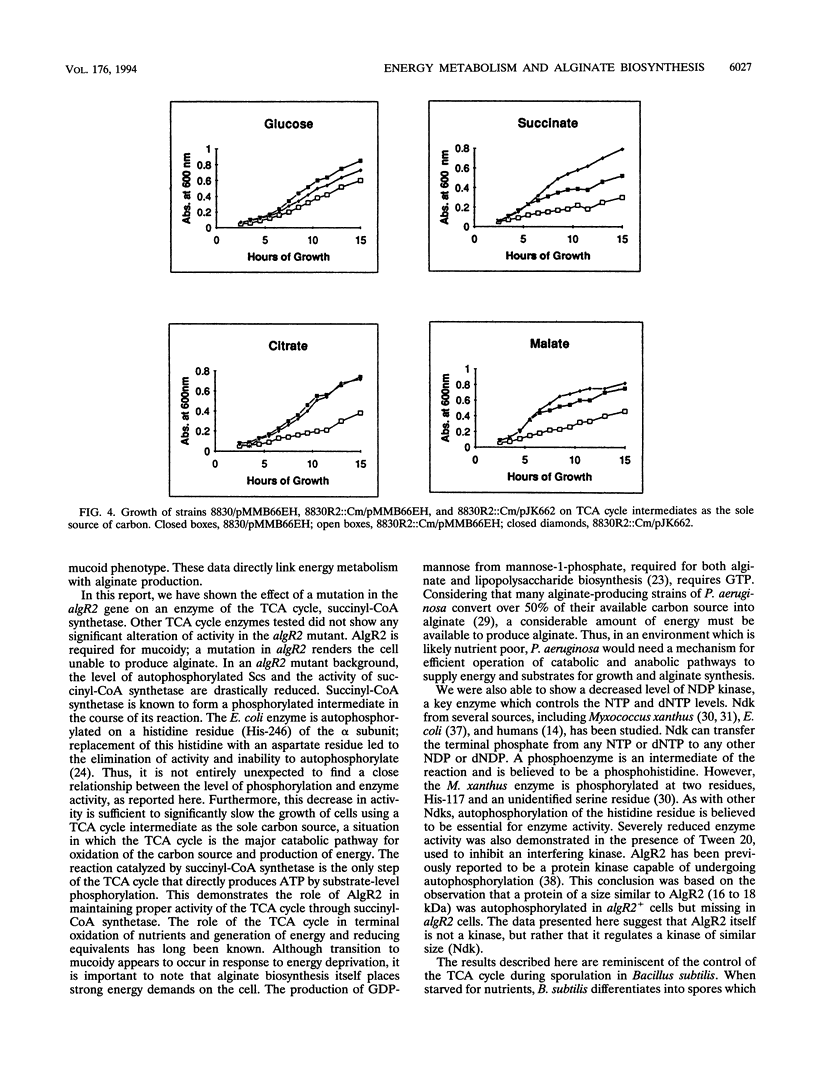
Abstract
Infection with mucoid, alginate-producing strains of Pseudomonas aeruginosa is the leading cause of mortality among patients with cystic fibrosis. Alginate production by P. aeruginosa is not constitutive but is triggered by stresses such as starvation. The algR2 (also termed algQ) gene has been previously identified as being necessary for mucoidy; an algR2 mutant strain is unable to produce alginate when grown at 37 degrees C. We show here that the levels of phosphorylated succinyl coenzyme A synthetase (Scs) and nucleoside diphosphate kinase (Ndk), which form a complex in P. aeruginosa, are reduced in the algR2 mutant. We were able to correlate the lower level of phosphorylated Scs with a decrease in Scs activity. Western blots (immunoblots) also showed a decreased level of Ndk in the algR2 mutant, but the presence of another kinase activity sensitive to Tween 20 provides the missing Ndk function. The effect of AlgR2 on tricarboxylic acid (TCA) cycle enzymes appears to be specific for Scs, since none of the other TCA cycle enzymes measured showed a significant decrease in activity. Furthermore, the ability of the algR2 mutant to grow on TCA cycle intermediates, but not glucose, is impaired. These data indicate that AlgR2 is responsible for maintaining proper operation of the TCA cycle and energy metabolism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Chan R., Lam J. S., Lam K., Costerton J. W. Influence of culture conditions on expression of the mucoid mode of growth of Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jan;19(1):8–16. doi: 10.1128/jcm.19.1.8-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992 May 8;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVault J. D., Kimbara K., Chakrabarty A. M. Pulmonary dehydration and infection in cystic fibrosis: evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol Microbiol. 1990 May;4(5):737–745. doi: 10.1111/j.1365-2958.1990.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Deretic V., Konyecsni W. M. Control of mucoidy in Pseudomonas aeruginosa: transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol. 1989 Jul;171(7):3680–3688. doi: 10.1128/jb.171.7.3680-3688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Martin D. W., Schurr M. J., Mudd M. H., Hibler N. S., Curcic R., Boucher J. C. Conversion to mucoidy in Pseudomonas aeruginosa. Biotechnology (N Y) 1993 Oct;11(10):1133–1136. doi: 10.1038/nbt1093-1133. [DOI] [PubMed] [Google Scholar]
- Dingman D. W., Rosenkrantz M. S., Sonenshein A. L. Relationship between aconitase gene expression and sporulation in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3068–3075. doi: 10.1128/jb.169.7.3068-3075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Ohman D. E. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J Bacteriol. 1988 Apr;170(4):1452–1460. doi: 10.1128/jb.170.4.1452-1460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Zielinski N. A., Chakrabarty A. M. Enhancer-like activity of A1gR1-binding site in alginate gene activation: positional, orientational, and sequence specificity. J Bacteriol. 1993 Sep;175(17):5452–5459. doi: 10.1128/jb.175.17.5452-5459.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Govan J. R. Alginate synthesis in mucoid Pseudomonas aeruginosa: a chromosomal locus involved in control. J Gen Microbiol. 1980 Aug;119(2):443–450. doi: 10.1099/00221287-119-2-443. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gilles A. M., Presecan E., Vonica A., Lascu I. Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem. 1991 May 15;266(14):8784–8789. [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F. A., Dawes E. A. Regulation of the tricarboxylic acid cycle and poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii grown under nitrogen or oxygen limitation. J Gen Microbiol. 1976 Dec;97(2):303–312. doi: 10.1099/00221287-97-2-303. [DOI] [PubMed] [Google Scholar]
- Kato J., Chu L., Kitano K., DeVault J. D., Kimbara K., Chakrabarty A. M., Misra T. K. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989 Dec 7;84(1):31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Kavanaugh-Black A., Connolly D. M., Chugani S. A., Chakrabarty A. M. Characterization of nucleoside-diphosphate kinase from Pseudomonas aeruginosa: complex formation with succinyl-CoA synthetase. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5883–5887. doi: 10.1073/pnas.91.13.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbara K., Hashimoto T., Fukuda M., Koana T., Takagi M., Oishi M., Yano K. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J Bacteriol. 1989 May;171(5):2740–2747. doi: 10.1128/jb.171.5.2740-2747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Phibbs P. V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- Lightfoot J., Lam J. S. Chromosomal mapping, expression and synthesis of lipopolysaccharide in Pseudomonas aeruginosa: a role for guanosine diphospho (GDP)-D-mannose. Mol Microbiol. 1993 May;8(4):771–782. doi: 10.1111/j.1365-2958.1993.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Majumdar R., Guest J. R., Bridger W. A. Functional consequences of substitution of the active site (phospho)histidine residue of Escherichia coli succinyl-CoA synthetase. Biochim Biophys Acta. 1991 Jan 8;1076(1):86–90. doi: 10.1016/0167-4838(91)90223-m. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Holloway B. W., Deretic V. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J Bacteriol. 1993 Feb;175(4):1153–1164. doi: 10.1128/jb.175.4.1153-1164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T. B., Chakrabarty A. M. Pseudomonas aeruginosa: genes and enzymes of alginate synthesis. Trends Microbiol. 1994 May;2(5):151–157. doi: 10.1016/0966-842x(94)90664-5. [DOI] [PubMed] [Google Scholar]
- May T. B., Shinabarger D., Maharaj R., Kato J., Chu L., DeVault J. D., Roychoudhury S., Zielinski N. A., Berry A., Rothmel R. K. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991 Apr;4(2):191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin L., Magnusson K., Rutberg L. Identification of the promoter of the Bacillus subtilis sdh operon. J Bacteriol. 1987 Jul;169(7):3232–3236. doi: 10.1128/jb.169.7.3232-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Dorado J., Almaula N., Inouye S., Inouye M. Autophosphorylation of nucleoside diphosphate kinase from Myxococcus xanthus. J Bacteriol. 1993 Feb;175(4):1176–1181. doi: 10.1128/jb.175.4.1176-1181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Dorado J., Inouye M., Inouye S. Nucleoside diphosphate kinase from Myxococcus xanthus. I. Cloning and sequencing of the gene. J Biol Chem. 1990 Feb 15;265(5):2702–2706. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Neidhardt F. C. Isolation and properties of a mutant of Escherichia coli with an insertional inactivation of the uspA gene, which encodes a universal stress protein. J Bacteriol. 1993 Jul;175(13):3949–3956. doi: 10.1128/jb.175.13.3949-3956.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Chakrabarty A. M. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun. 1981 Jul;33(1):142–148. doi: 10.1128/iai.33.1.142-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Ray N. B., Mathews C. K. Nucleoside diphosphokinase: a functional link between intermediary metabolism and nucleic acid synthesis. Curr Top Cell Regul. 1992;33:343–357. doi: 10.1016/b978-0-12-152833-1.50025-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury S., Sakai K., Schlictman D., Chakrabarty A. M. Signal transduction in exopolysaccharide alginate synthesis: phosphorylation of the response regulator AlgR1 in Pseudomonas aeruginosa and Escherichia coli. Gene. 1992 Mar 1;112(1):45–51. doi: 10.1016/0378-1119(92)90301-5. [DOI] [PubMed] [Google Scholar]
- Saeki T., Hori M., Umezawa H. Pyruvate kinase of Escherichia coli. Its role in supplying nucleoside triphosphates in cells under anaerobic conditions. J Biochem. 1974 Sep;76(3):631–637. doi: 10.1093/oxfordjournals.jbchem.a130607. [DOI] [PubMed] [Google Scholar]
- Sokol P. A., Luan M. Z., Storey D. G., Thirukkumaran P. Genetic rearrangement associated with in vivo mucoid conversion of Pseudomonas aeruginosa PAO is due to insertion elements. J Bacteriol. 1994 Feb;176(3):553–562. doi: 10.1128/jb.176.3.553-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry J. M., Piña S. E., Mattingly S. J. Environmental conditions which influence mucoid conversion Pseudomonas aeruginosa PAO1. Infect Immun. 1991 Feb;59(2):471–477. doi: 10.1128/iai.59.2.471-477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry J. M., Piña S. E., Mattingly S. J. Role of energy metabolism in conversion of nonmucoid Pseudomonas aeruginosa to the mucoid phenotype. Infect Immun. 1992 Apr;60(4):1329–1335. doi: 10.1128/iai.60.4.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Enzymatic control of the metabolic activity of Pseudomonas aeruginosa grown in glucose or succinate media. Biochim Biophys Acta. 1969 Dec 30;192(3):395–401. doi: 10.1016/0304-4165(69)90388-2. [DOI] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Utilization of dicarboxylic acids by Pseudomonas aeruginosa. Can J Microbiol. 1969 Sep;15(9):1095–1100. doi: 10.1139/m69-194. [DOI] [PubMed] [Google Scholar]
- Uratani-Wong B., Lopez J. M., Freese E. Induction of citric acid cycle enzymes during initiation of sporulation by guanine nucleotide deprivation. J Bacteriol. 1981 Apr;146(1):337–344. doi: 10.1128/jb.146.1.337-344.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Sokol P. A., Bryan L. E., Storey D. G., Mattingly S. J., Vogel H. J., Ceri H. In vivo regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis. 1991 Jan;163(1):143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]
- Zielinski N. A., Maharaj R., Roychoudhury S., Danganan C. E., Hendrickson W., Chakrabarty A. M. Alginate synthesis in Pseudomonas aeruginosa: environmental regulation of the algC promoter. J Bacteriol. 1992 Dec;174(23):7680–7688. doi: 10.1128/jb.174.23.7680-7688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]