Abstract
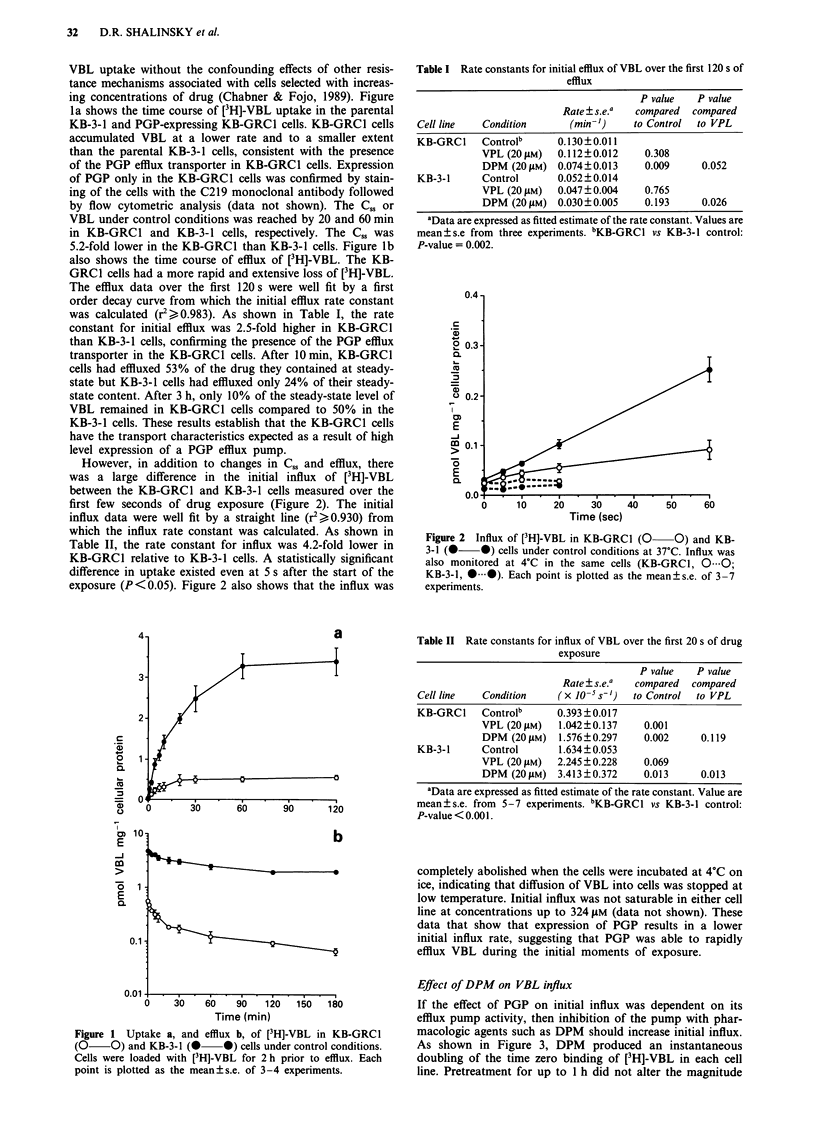
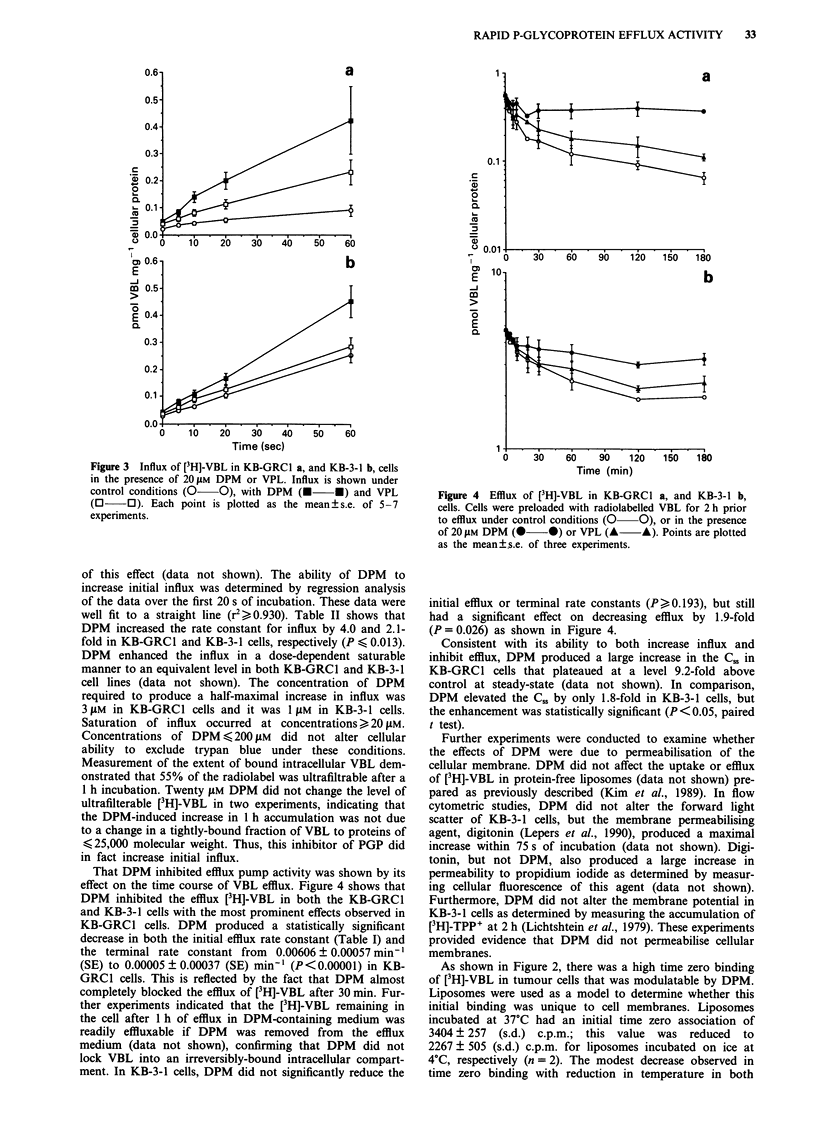
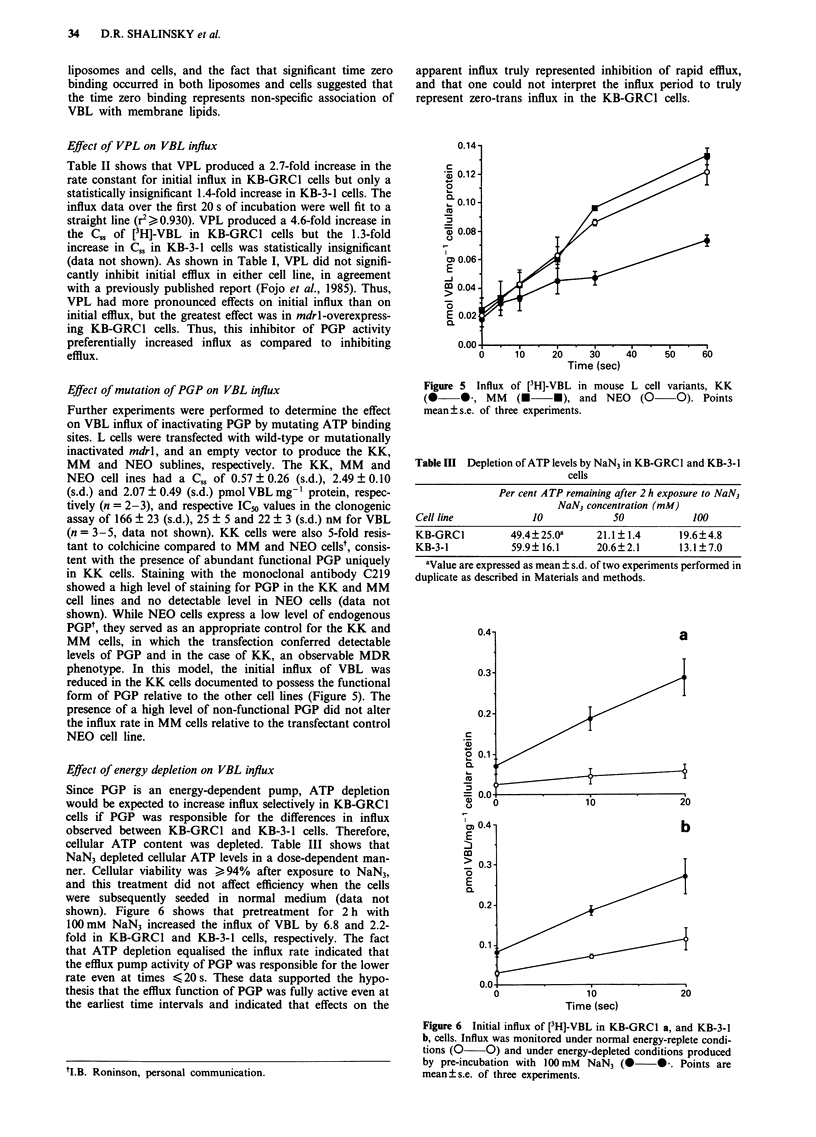
P-glycoprotein (PGP) is an energy-dependent efflux pump that serves to protect cells against the cytotoxicity of many natural product drugs including vinblastine (VBL). In this study we investigated the role of PGP in regulating initial VBL influx. The apparent influx of VBL, measured over the first 20 s, was 2-fold lower in KB-GRC1 cells expressing a transfected mdr1 gene at high level than in non-expressing parental KB-3-1 cells. Inhibition of PGP efflux function with dipyridamole increased the influx rate constant by 4.0-fold in the KB-GRC1 cells but only 2.1-fold in the KB-3-1 cells. Verapamil, another inhibitor of PGP-mediated efflux, increased the initial influx rate constant by 2.7-fold in the KB-GRC1 cells but only 1.4-fold in the KB-3-1 cells. Inhibition of PGP function by depletion of ATP increased influx by 6.8-fold and 2.2-fold in the two cell types, respectively. Mutation of PGP at both ATP binding sites abolished its ability to limit initial influx. Thus, VBL is serving as an efficient substrate for the efflux pump even within the first few seconds of drug exposure, consistent with the hypothesis that PGP may directly efflux drug from the cell membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Asoh K., Saburi Y., Sato S., Nogae I., Kohno K., Kuwano M. Potentiation of some anticancer agents by dipyridamole against drug-sensitive and drug-resistant cancer cell lines. Jpn J Cancer Res. 1989 May;80(5):475–481. doi: 10.1111/j.1349-7006.1989.tb02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Cano-Gauci D. F., Busche R., Tümmler B., Riordan J. R. Fast kinetic analysis of drug transport in multidrug resistant cells using a pulsed quench-flow apparatus. Biochem Biophys Res Commun. 1990 Feb 28;167(1):48–53. doi: 10.1016/0006-291x(90)91728-b. [DOI] [PubMed] [Google Scholar]
- Chabner B. A., Fojo A. Multidrug resistance: P-glycoprotein and its allies--the elusive foes. J Natl Cancer Inst. 1989 Jun 21;81(12):910–913. doi: 10.1093/jnci/81.12.910. [DOI] [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991 Jul 12;66(1):85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Slovak M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989 Jan;59(1):42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffie A. M., Alam T., Seneviratne C., Beenken S. W., Batra J. K., Shea T. C., Henner W. D., Goldenberg G. J. Multifactorial resistance to adriamycin: relationship of DNA repair, glutathione transferase activity, drug efflux, and P-glycoprotein in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1988 Jul 1;48(13):3595–3602. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Greenberger L. M., Lisanti C. J., Silva J. T., Horwitz S. B. Domain mapping of the photoaffinity drug-binding sites in P-glycoprotein encoded by mouse mdr1b. J Biol Chem. 1991 Nov 5;266(31):20744–20751. [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Dhir R., Croop J., Talbot F. A single amino acid substitution strongly modulates the activity and substrate specificity of the mouse mdr1 and mdr3 drug efflux pumps. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7289–7293. doi: 10.1073/pnas.88.16.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait W. N., Aftab D. T. Rational design and pre-clinical pharmacology of drugs for reversing multidrug resistance. Biochem Pharmacol. 1992 Jan 9;43(1):103–107. doi: 10.1016/0006-2952(92)90667-8. [DOI] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Leach F. R., Webster J. J. Commercially available firefly luciferase reagents. Methods Enzymol. 1986;133:51–70. doi: 10.1016/0076-6879(86)33055-6. [DOI] [PubMed] [Google Scholar]
- Lepers A., Cacan R., Verbert A. Permeabilized cells as a way of gaining access to intracellular organelles: an approach to glycosylation reactions. Biochimie. 1990 Jan;72(1):1–5. doi: 10.1016/0300-9084(90)90166-e. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M., Tsuruo T. Competitive inhibition by verapamil of ATP-dependent high affinity vincristine binding to the plasma membrane of multidrug-resistant K562 cells without calcium ion involvement. Cancer Res. 1989 Mar 15;49(6):1452–1455. [PubMed] [Google Scholar]
- Ramu A., Pollard H. B., Rosario L. M. Doxorubicin resistance in P388 leukemia--evidence for reduced drug influx. Int J Cancer. 1989 Sep 15;44(3):539–547. doi: 10.1002/ijc.2910440328. [DOI] [PubMed] [Google Scholar]
- Raviv Y., Pollard H. B., Bruggemann E. P., Pastan I., Gottesman M. M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J Biol Chem. 1990 Mar 5;265(7):3975–3980. [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Safa A. R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalinsky D. R., Andreeff M., Howell S. B. Modulation of drug sensitivity by dipyridamole in multidrug resistant tumor cells in vitro. Cancer Res. 1990 Dec 1;50(23):7537–7543. [PubMed] [Google Scholar]
- Shalinsky D. R., Slovak M. L., Howell S. B. Modulation of vinblastine sensitivity by dipyridamole in multidrug resistant fibrosarcoma cells lacking mdr1 expression. Br J Cancer. 1991 Oct;64(4):705–709. doi: 10.1038/bjc.1991.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Roninson I. B., Chin J. E., Soffir R., Pastan I., Gottesman M. M. Multidrug resistance of DNA-mediated transformants is linked to transfer of the human mdr1 gene. Mol Cell Biol. 1986 Nov;6(11):4039–4045. doi: 10.1128/mcb.6.11.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmaiah K. N., Sethi V. S. Chemical characterization of the degradation products of vinblastine dihydrogen sulfate. Cancer Res. 1985 Nov;45(11 Pt 1):5382–5385. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]


