Abstract
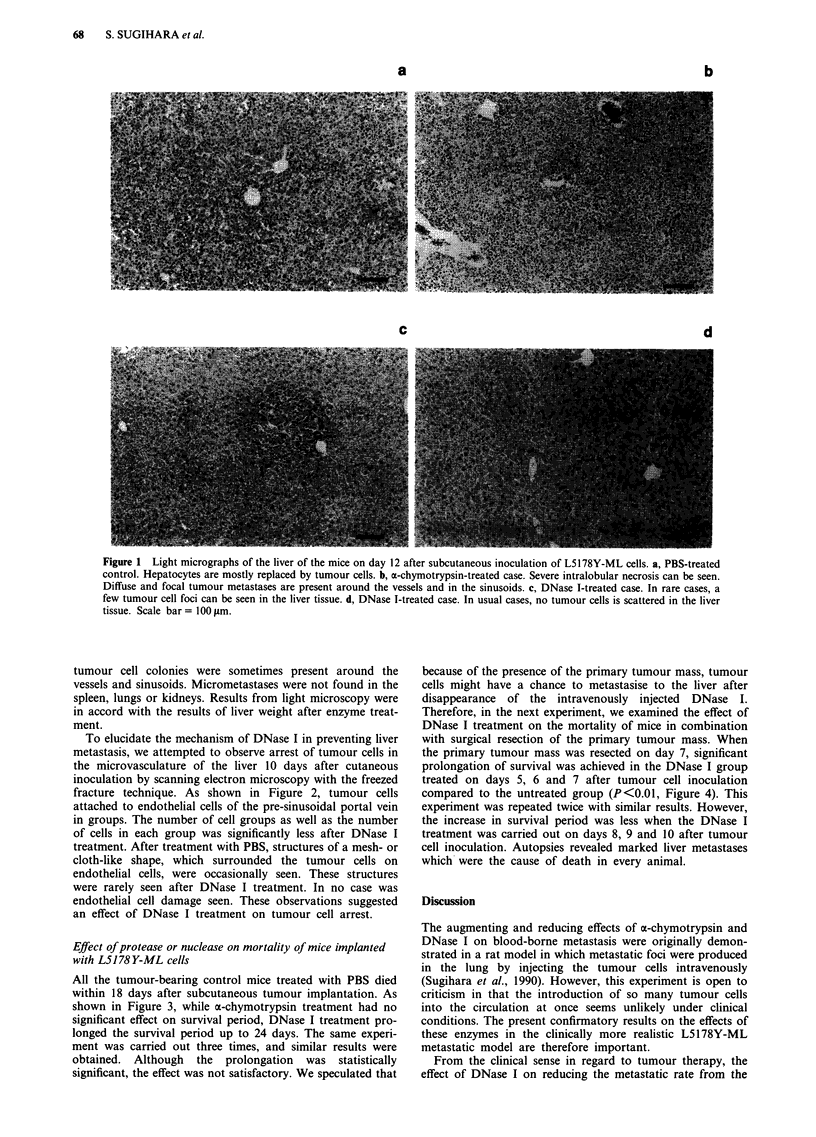
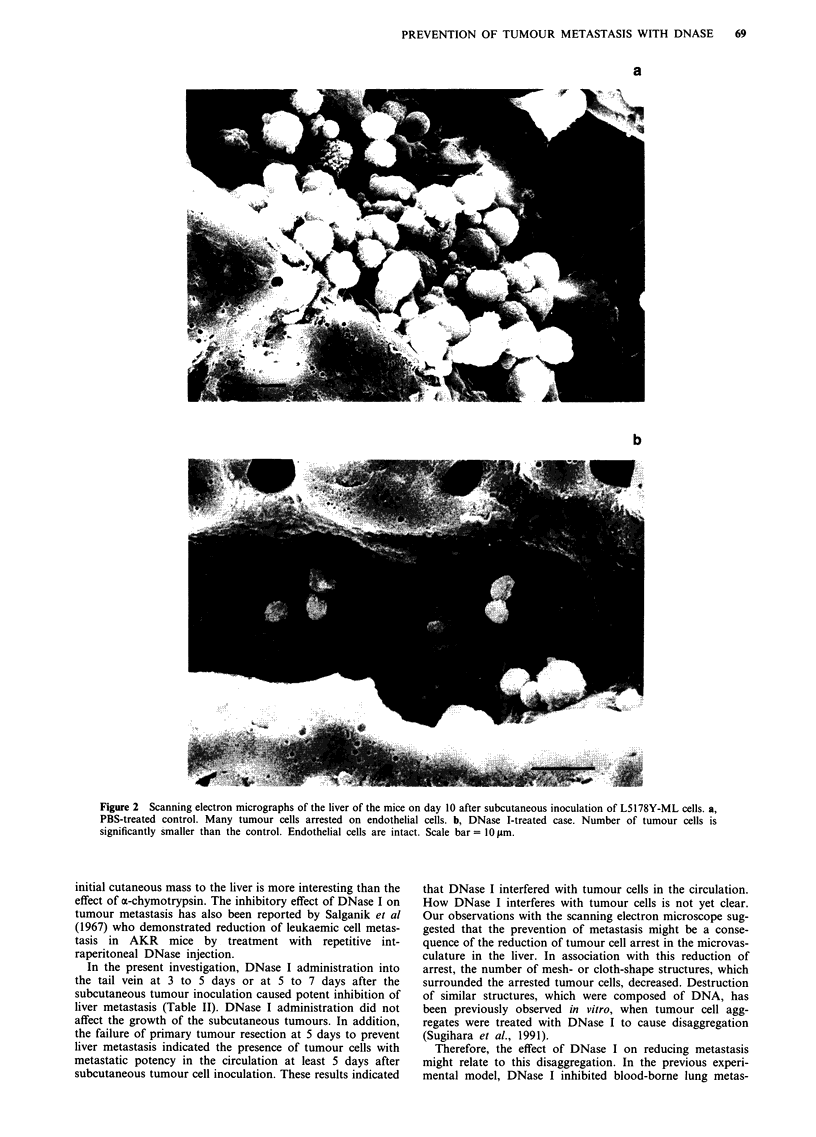
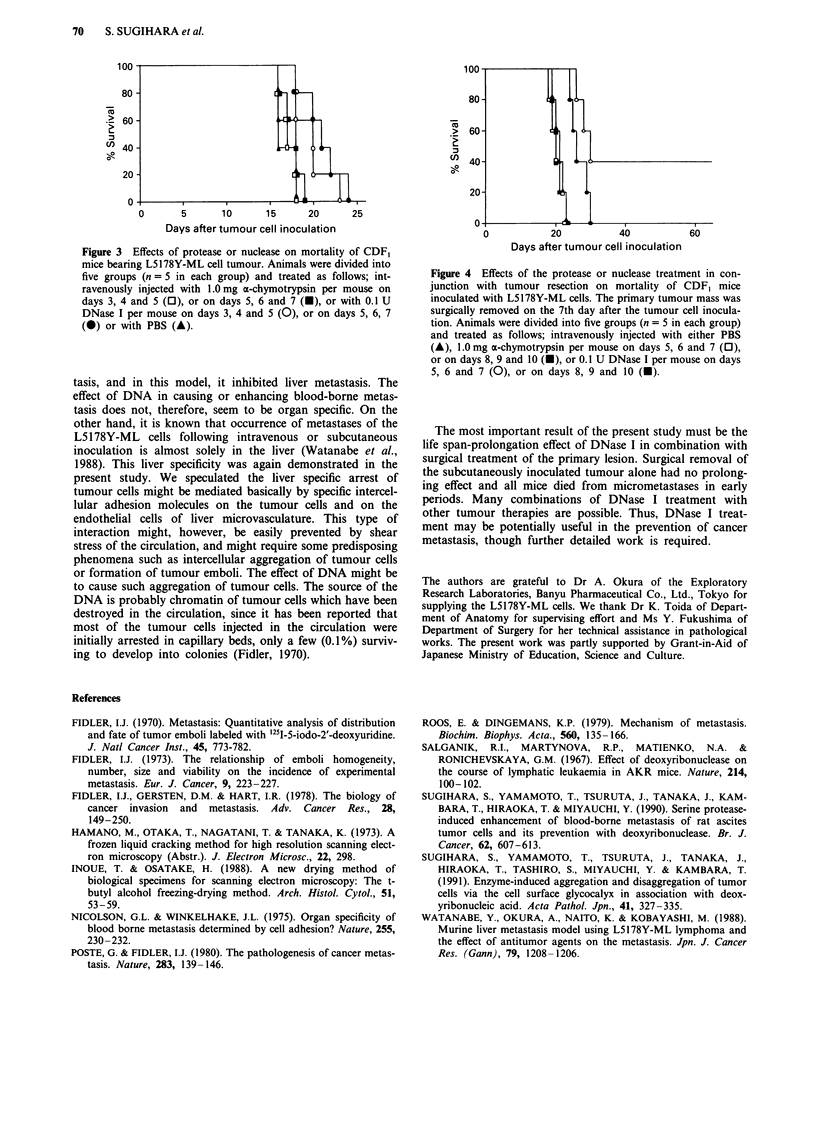
Murine L5178Y-ML cells, when transplanted subcutaneously into the flank of (BALB/c x DBA/2)F1 mice, grew locally and always formed spontaneous metastases in the liver. Even after surgical removal of the primary tumour mass 5 or 7 days after tumour cell inoculation, all mice died due to liver metastases within 18 days. Using this model of tumour metastasis, we examined whether serine protease or deoxyribonuclease I (DNase I) would affect metastasis. Spontaneous liver metastasis of L5178Y-ML cells was enhanced by systemic administration of alpha-chymotrypsin at 3, 4 and 5 days or at 5, 6 and 7 days after tumour cell inoculation. This result was consistent with a previous report on blood-borne lung metastasis. In contrast, systemic administration of DNase I at 3, 4 and 5 days or at 5, 6 and 7 days after tumour cell inoculation inhibited liver metastasis. Neither treatment affected primary tumour growth. An influence of DNase I on tumour cell arrest in the microvasculature of the liver was suggested by scanning electron microscopy. DNase I treatment resulted in a statistically significant prolongation of the survival period, however, the effect was not satisfactory. A more striking anti-metastatic treatment resulting in a greater prolongation of the survival period was achieved by combining surgical removal of the primary tumour mass with DNase I treatment. These results suggest that DNase I could be a potential therapeutic agent used in conjunction with surgery to prevent clinical blood-borne metastasis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973 Mar;9(3):223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Inoué T., Osatake H. A new drying method of biological specimens for scanning electron microscopy: the t-butyl alcohol freeze-drying method. Arch Histol Cytol. 1988 Mar;51(1):53–59. doi: 10.1679/aohc.51.53. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Winkelhake J. L. Organ specificity of blood-borne tumour metastasis determined by cell adhesion? Nature. 1975 May 15;255(5505):230–232. doi: 10.1038/255230a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Roos E., Dingemans K. P. Mechanisms of metastasis. Biochim Biophys Acta. 1979 Feb 4;560(1):135–166. doi: 10.1016/0304-419x(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Salganik R. I., Martynova R. P., Matienko N. A., Ronichevskaya G. M. Effect of deoxyribonuclease on the course of lymphatic leukaemia in AKR mice. Nature. 1967 Apr 1;214(5083):100–102. doi: 10.1038/214100a0. [DOI] [PubMed] [Google Scholar]
- Sugihara S., Yamamoto T., Tsuruta J., Tanaka J., Hiraoka T., Tashiro S., Miyauchi Y., Kambara T. Enzyme-induced aggregation and disaggregation of tumor cells via the cell surface glycocalyx in association with deoxyribonucleic acid. Acta Pathol Jpn. 1991 May;41(5):327–335. doi: 10.1111/j.1440-1827.1991.tb01655.x. [DOI] [PubMed] [Google Scholar]
- Sugihara S., Yamamoto T., Tsuruta J., Tanaka J., Kambara T., Hiraoka T., Miyauchi Y. Serine protease-induced enhancement of blood-borne metastasis of rat ascites tumour cells and its prevention with deoxyribonuclease. Br J Cancer. 1990 Oct;62(4):607–613. doi: 10.1038/bjc.1990.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Okura A., Naito K., Kobayashi M. Murine liver metastasis model using L5178Y-ML lymphoma and the effect of antitumor agents on the metastasis. Jpn J Cancer Res. 1988 Nov;79(11):1208–1216. doi: 10.1111/j.1349-7006.1988.tb01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]