Abstract
Nicotinamide (NA) and metoclopramide (MCA) have been shown to be sensitisers of the effects of radiation and drugs in experimental rodent tumours growing in skin and muscle. We have used 86Rb uptake to investigate the effects of these two drugs on the distribution of blood to a mouse carcinoma (NT) growing in skin, muscle or the gut wall, as well as to the host normal tissue. NA caused an increase in cardiac output distribution (COD) of between 17 and 92% to tumours in the three sites. When this increase is related to the changes in COD to the host normal tissues, however, COD to tumours in skin and muscle was increased by a factor of 1.8 and to tumours in the gut wall by a factor of 1.7. MCA caused a consistent increase in COD to tumours growing in muscle, but the effects in tumours in skin and gut were variable with time. Again when related to the change in COD to host normal tissues, a factor of 2.1 was seen for COD to tumours growing in muscle and gut. Both NA and MCA alter COD to tumours in some sites relative to host tissues in a way that could enhance anti-cancer drug delivery to tumours, though the effects of NA are more reliable in our systems.
Full text
PDF
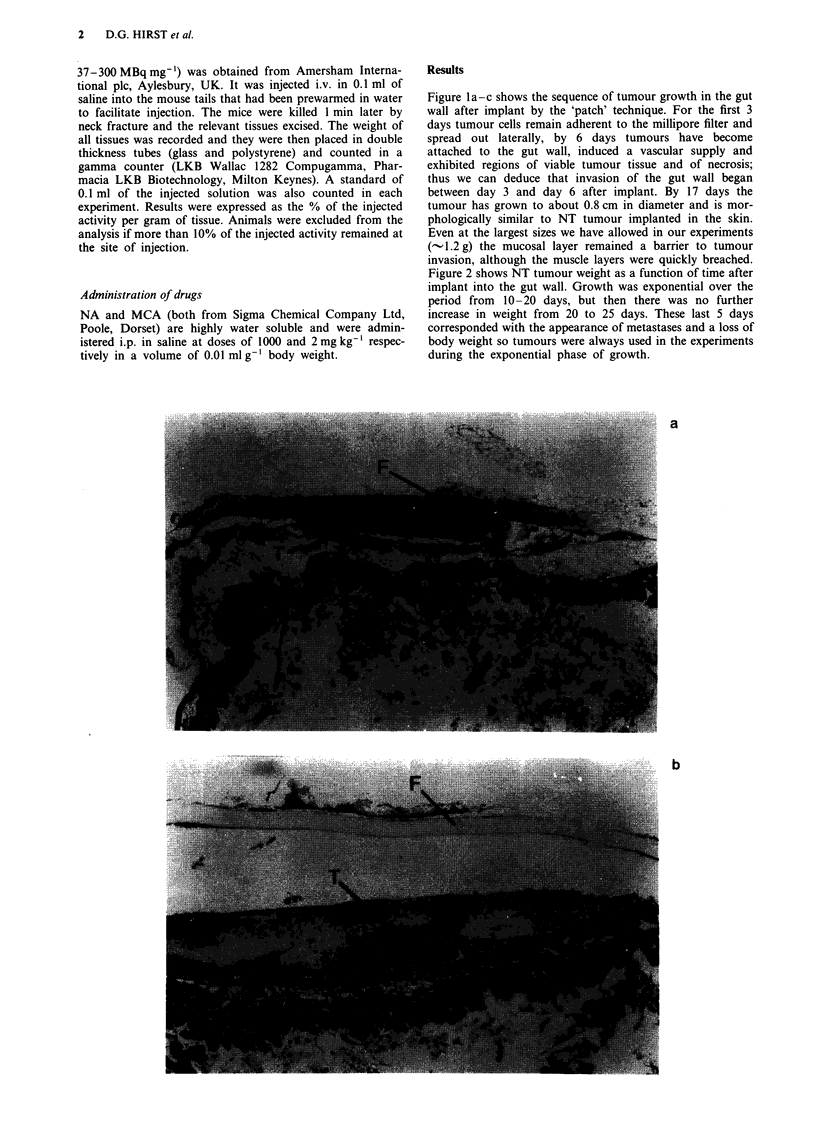
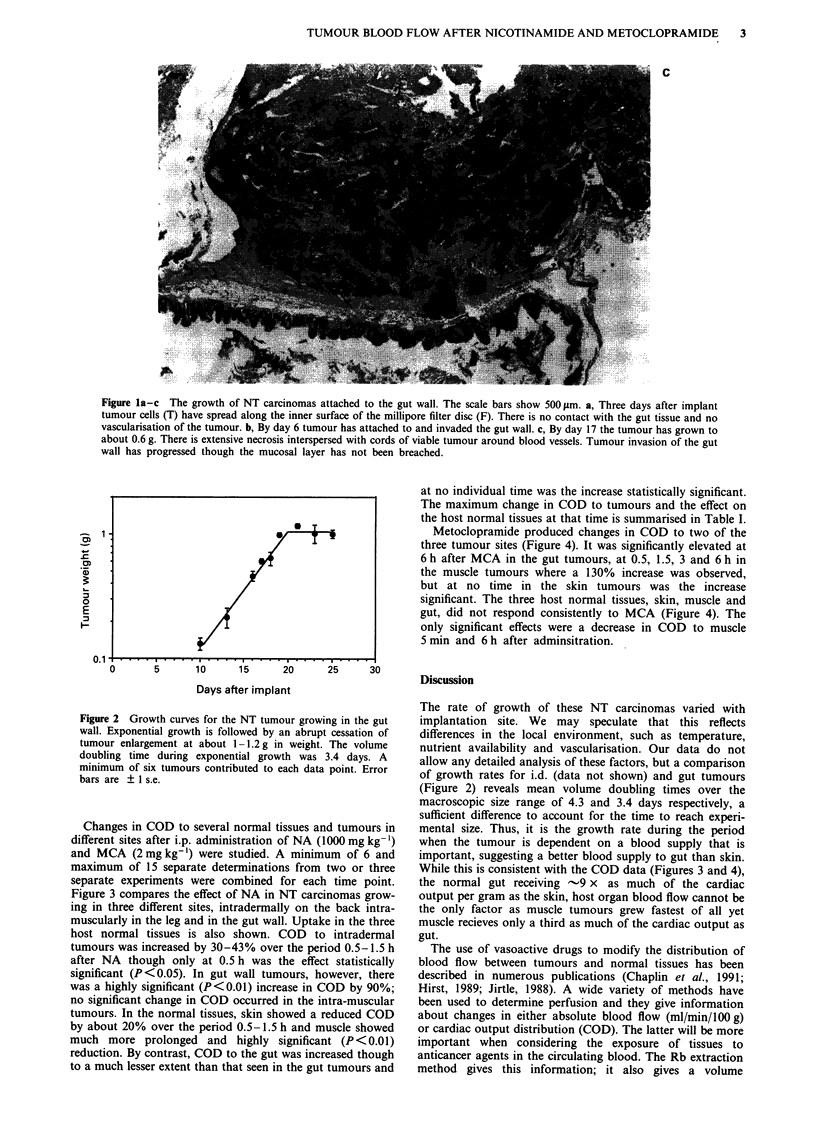
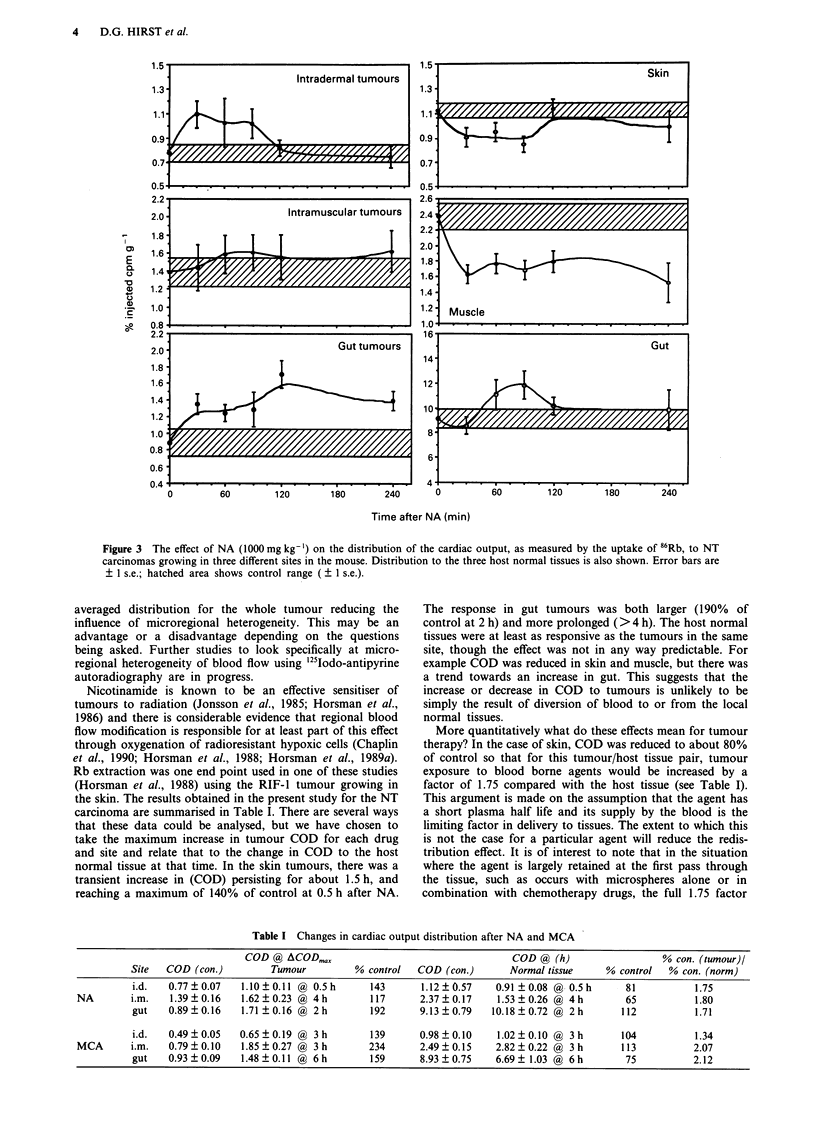


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hur E., Chen C. C., Elkind M. M. Inhibitors of poly(adenosine diphosphoribose) synthetase, examination of metabolic perturbations, and enhancement of radiation response in Chinese hamster cells. Cancer Res. 1985 May;45(5):2123–2127. [PubMed] [Google Scholar]
- Chaplin D. J., Horsman M. R., Trotter M. J. Effect of nicotinamide on the microregional heterogeneity of oxygen delivery within a murine tumor. J Natl Cancer Inst. 1990 Apr 18;82(8):672–676. doi: 10.1093/jnci/82.8.672. [DOI] [PubMed] [Google Scholar]
- Gralla R. J., Itri L. M., Pisko S. E., Squillante A. E., Kelsen D. P., Braun D. W., Jr, Bordin L. A., Braun T. J., Young C. W. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med. 1981 Oct 15;305(16):905–909. doi: 10.1056/NEJM198110153051601. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Brown D. M., Lemmon M. J., Brown J. M., Lee W. W. Preferential tumor radiosensitization by analogs of nicotinamide and benzamide. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1307–1310. doi: 10.1016/0360-3016(86)90160-4. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Brown J. M., Hirst V. K., Lemmon M. J., Wood P. J., Dunphy E. P., Overgaard J. Mechanism of action of the selective tumor radiosensitizer nicotinamide. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):685–690. doi: 10.1016/0360-3016(88)90312-4. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Chaplin D. J., Brown J. M. Tumor radiosensitization by nicotinamide: a result of improved perfusion and oxygenation. Radiat Res. 1989 Apr;118(1):139–150. [PubMed] [Google Scholar]
- Horsman M. R., Overgaard J., Christensen K. L., Trotter M. J., Chaplin D. J. Mechanism for the reduction of tumour hypoxia by nicotinamide and the clinical relevance for radiotherapy. Biomed Biochim Acta. 1989;48(2-3):S251–S254. [PubMed] [Google Scholar]
- Jirtle R. L. Chemical modification of tumour blood flow. Int J Hyperthermia. 1988 Jul-Aug;4(4):355–371. doi: 10.3109/02656738809016490. [DOI] [PubMed] [Google Scholar]
- Jonsson G. G., Kjellén E., Pero R. W., Cameron R. Radiosensitization effects of nicotinamide on malignant and normal mouse tissue. Cancer Res. 1985 Aug;45(8):3609–3614. [PubMed] [Google Scholar]
- Kjellén E., Wennerberg J., Pero R. Metoclopramide enhances the effect of cisplatin on xenografted squamous cell carcinoma of the head and neck. Br J Cancer. 1989 Feb;59(2):247–250. doi: 10.1038/bjc.1989.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybak S., Kjellén E., Wennerberg J., Pero R. Metoclopramide enhances the effect of ionizing radiation on xenografted squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1990 Dec;19(6):1419–1424. doi: 10.1016/0360-3016(90)90353-l. [DOI] [PubMed] [Google Scholar]
- Lybak S., Pero R. W. The benzamide derivative metoclopramide causes DNA damage and inhibition of DNA repair in human peripheral mononuclear leukocytes at clinically relevant doses. Carcinogenesis. 1991 Sep;12(9):1613–1617. doi: 10.1093/carcin/12.9.1613. [DOI] [PubMed] [Google Scholar]
- Lybak S., Wennerberg J., Kjellén E., Pero R. W. Dose schedule evaluation of metoclopramide as a potentiator of cisplatin and carboplatin treatments of xenografted squamous cell carcinomas of the head and neck. Anticancer Drugs. 1991 Aug;2(4):375–382. doi: 10.1097/00001813-199108000-00007. [DOI] [PubMed] [Google Scholar]
- SAPIRSTEIN L. A. Regional blood flow by fractional distribution of indicators. Am J Physiol. 1958 Apr;193(1):161–168. doi: 10.1152/ajplegacy.1958.193.1.161. [DOI] [PubMed] [Google Scholar]





