Abstract
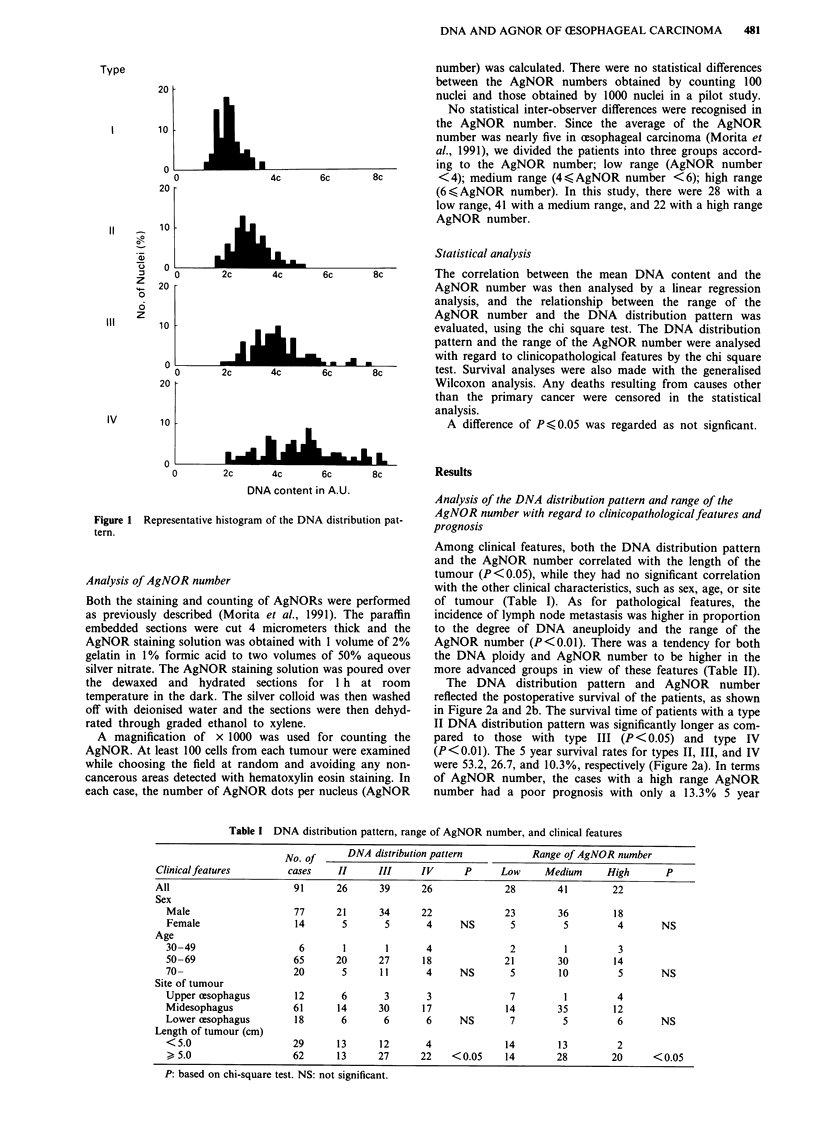
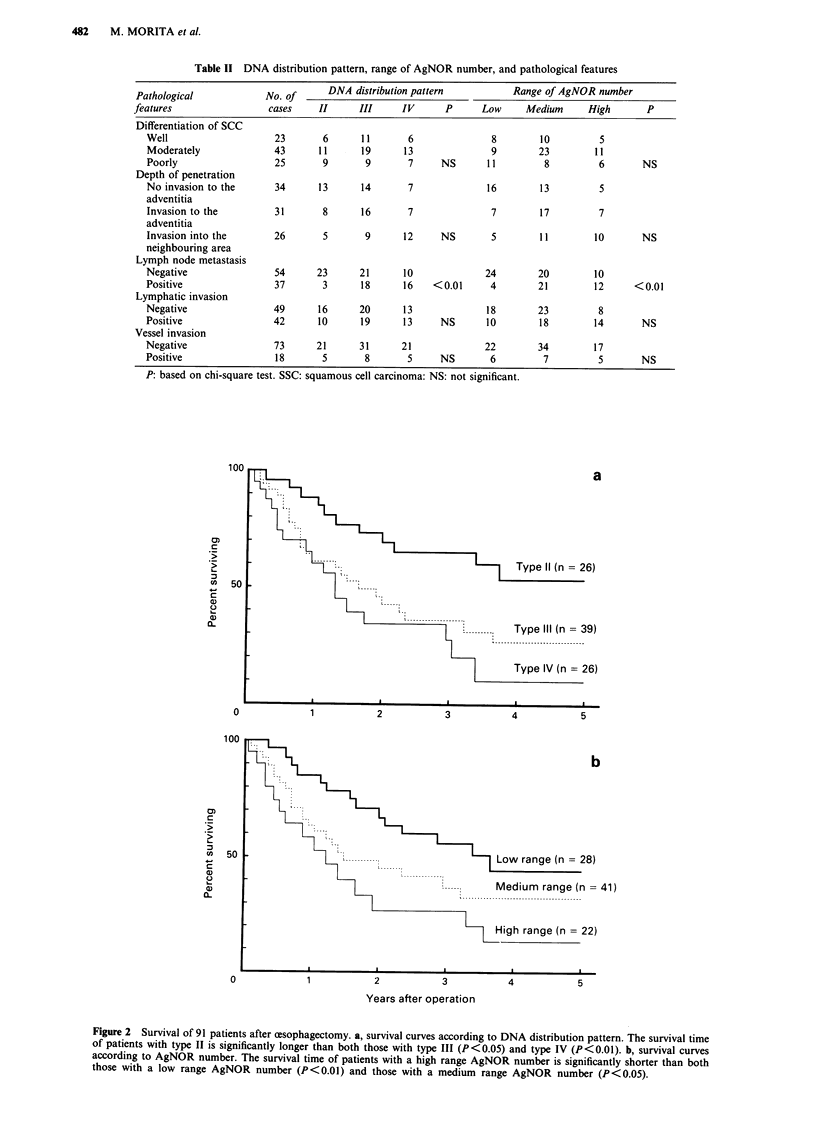
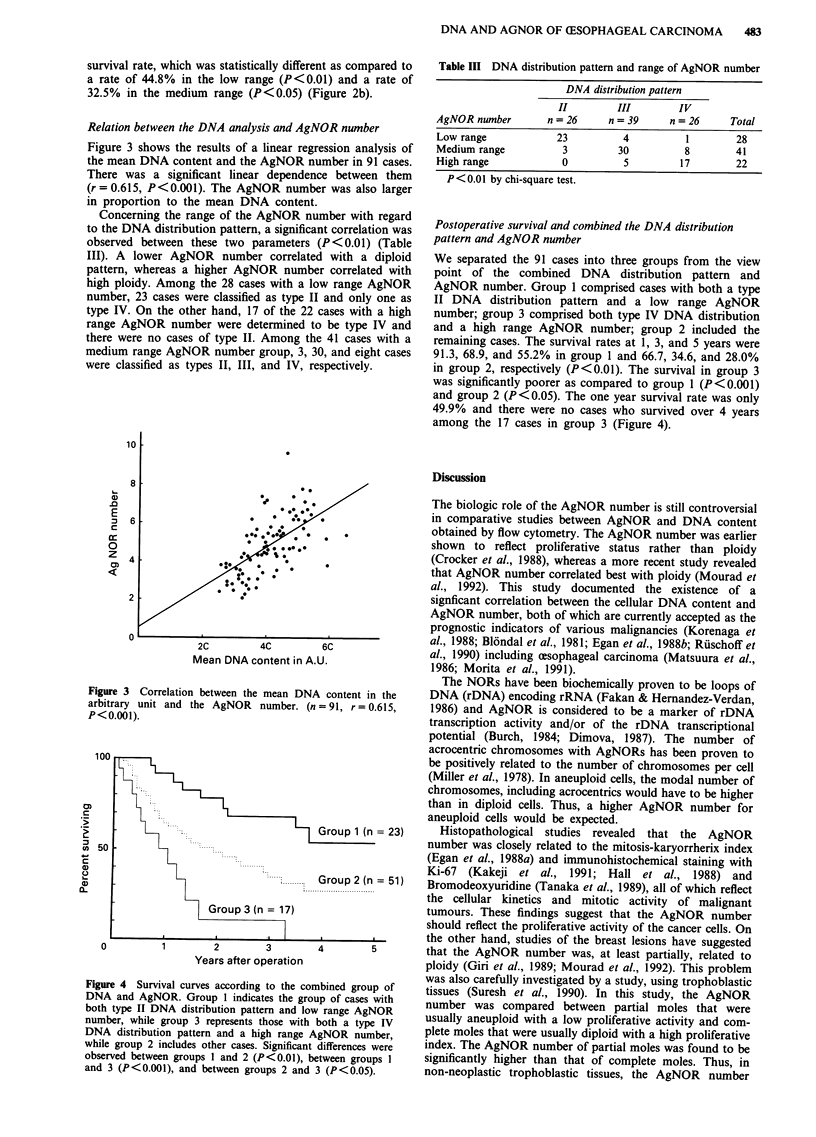
The cytophotometric DNA content and the argyrophilic nucleolar organiser regions (AgNORs) of biopsy specimens taken before undergoing any treatment were examined in 91 surgically treated oesophageal carcinoma cases. There was a significant linear dependence between the mean DNA content and the number of AgNOR per nucleus (AgNOR number) (r = 0.615, P < 0.001). The DNA distribution pattern and the range of the AgNOR number also showed a significant correlation (P < 0.01). Twenty three of 28 cases with a low AgNOR number (< 4) were then determined to have a diploid pattern (type II), while 17 out of 22 cases with a high AgNOR number (> or = 6) had high ploidy values (type IV). The patients with a type II DNA distribution pattern and a low AgNOR number thus showed a good post-operative course with a 5 year survival rate of 55.2%, whereas no patients survived over 4 years among the 17 cases with both a type IV DNA pattern and a high AgNOR number (P < 0.001). These data thus demonstrate the close relationship between cytophotometric DNA content and AgNOR number and suggest that the combined detection of these two parameters, using biopsy specimens, should be of benefit in making an accurate preoperative evaluation of prognosis for patients with oesophageal carcinoma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Ogura S., Kunikane H., Suko N., Watanabe N., Nakajima I., Kawakami Y., Inoue K. Nucleolar organizer regions in precancerous and cancerous lesions of the bronchus. Cancer. 1991 Jan 15;67(2):472–475. doi: 10.1002/1097-0142(19910115)67:2<472::aid-cncr2820670225>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Blöndal T., Bengtsson A. Nuclear DNA measurements in squamous cell carcinoma of the lung: a guide for prognostic evaluation. Anticancer Res. 1981;1(2):79–86. [PubMed] [Google Scholar]
- Böcking A., Auffermann W., Vogel H., Schlöndorff G., Goebbels R. Diagnosis and grading of malignancy in squamous epithelial lesions of the larynx with DNA cytophotometry. Cancer. 1985 Oct 1;56(7):1600–1604. doi: 10.1002/1097-0142(19851001)56:7<1600::aid-cncr2820560723>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Crocker J., Macartney J. C., Smith P. J. Correlation between DNA flow cytometric and nucleolar organizer region data in non-Hodgkin's lymphomas. J Pathol. 1988 Feb;154(2):151–156. doi: 10.1002/path.1711540207. [DOI] [PubMed] [Google Scholar]
- Dimova R. N., Markov D. V., Gajdardjieva K. C., Dabeva M. D., Hadjiolov A. A. Electron microscopic localization of silver staining NOR-proteins in rat liver nucleoli upon D-galactosamine block of transcription. Eur J Cell Biol. 1982 Oct;28(2):272–277. [PubMed] [Google Scholar]
- Egan M., Raafat F., Crocker J., Williams D. Comparative study of the degree of differentiation of neuroblastoma and mean numbers of nucleolar organiser regions. J Clin Pathol. 1988 May;41(5):527–531. doi: 10.1136/jcp.41.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Hernandez-Verdun D. The nucleolus and the nucleolar organizer regions. Biol Cell. 1986;56(3):189–205. doi: 10.1111/j.1768-322x.1986.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Giri D. D., Nottingham J. F., Lawry J., Dundas S. A., Underwood J. C. Silver-binding nucleolar organizer regions (AgNORs) in benign and malignant breast lesions: correlations with ploidy and growth phase by DNA flow cytometry. J Pathol. 1989 Apr;157(4):307–313. doi: 10.1002/path.1711570407. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Crocker J., Watts A., Stansfeld A. G. A comparison of nucleolar organizer region staining and Ki-67 immunostaining in non-Hodgkin's lymphoma. Histopathology. 1988 Apr;12(4):373–381. doi: 10.1111/j.1365-2559.1988.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Howell W. M., Black D. A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980 Aug 15;36(8):1014–1015. doi: 10.1007/BF01953855. [DOI] [PubMed] [Google Scholar]
- Japanese Society for Esophageal Diseases Guide lines for the clinical and pathologic studies on carcinoma of the esophagus. Jpn J Surg. 1976 Jun;6(2):69–78. doi: 10.1007/BF02468889. [DOI] [PubMed] [Google Scholar]
- Kakeji Y., Korenaga D., Tsujitani S., Haraguchi M., Maehara Y., Sugimachi K. Predictive value of Ki-67 and argyrophilic nucleolar organizer region staining for lymph node metastasis in gastric cancer. Cancer Res. 1991 Jul 1;51(13):3503–3506. [PubMed] [Google Scholar]
- Korenaga D., Okamura T., Saito A., Baba H., Sugimachi K. DNA ploidy is closely linked to tumor invasion, lymph node metastasis, and prognosis in clinical gastric cancer. Cancer. 1988 Jul 15;62(2):309–313. doi: 10.1002/1097-0142(19880715)62:2<309::aid-cncr2820620214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Korenaga D., Saito A., Baba H., Watanabe A., Okamura T., Maehara Y., Sugimachi K. Cytophotometrically determined DNA content, mitotic activity, and lymph node metastasis in clinical gastric cancer. Surgery. 1990 Mar;107(3):262–267. [PubMed] [Google Scholar]
- Matsuura H., Kuwano H., Morita M., Tsutsui S., Kido Y., Mori M., Sugimachi K. Predicting recurrence time of esophageal carcinoma through assessment of histologic factors and DNA ploidy. Cancer. 1991 Mar 1;67(5):1406–1411. doi: 10.1002/1097-0142(19910301)67:5<1406::aid-cncr2820670522>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Sugimachi K., Ueo H., Kuwano H., Koga Y., Okamura T. Malignant potentiality of squamous cell carcinoma of the esophagus predictable by DNA analysis. Cancer. 1986 May 1;57(9):1810–1814. doi: 10.1002/1097-0142(19860501)57:9<1810::aid-cncr2820570917>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Dev V. G., Tantravahi R., Croce C. M., Miller O. J. Human tumor and rodent-human hybrid cells with an increased number of active human NORs. Cytogenet Cell Genet. 1978;21(1-2):33–41. doi: 10.1159/000130876. [DOI] [PubMed] [Google Scholar]
- Morita M., Kuwano H., Matsuda H., Moriguchi S., Sugimachi K. Prognostic significance of argyrophilic nucleolar organizer regions in esophageal carcinoma. Cancer Res. 1991 Oct 1;51(19):5339–5341. [PubMed] [Google Scholar]
- Mourad W. A., Erkman-Balis B., Livingston S., Shoukri M., Cox C. E., Nicosia S. V., Rowlands D. T., Jr Argyrophilic nucleolar organizer regions in breast carcinoma. Correlation with DNA flow cytometry, histopathology, and lymph node status. Cancer. 1992 Apr 1;69(7):1739–1744. doi: 10.1002/1097-0142(19920401)69:7<1739::aid-cncr2820690715>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ofner D., Tötsch M., Sandbichler P., Hallbrucker C., Margreiter R., Mikuz G., Schmid K. W. Silver stained nucleolar organizer region proteins (Ag-NORs) as a predictor of prognosis in colonic cancer. J Pathol. 1990 Sep;162(1):43–49. doi: 10.1002/path.1711620109. [DOI] [PubMed] [Google Scholar]
- PATAU K. Absorption microphotometry of irregular-shaped objects. Chromosoma. 1952;5(4):341–362. doi: 10.1007/BF01271492. [DOI] [PubMed] [Google Scholar]
- Ploton D., Menager M., Jeannesson P., Himber G., Pigeon F., Adnet J. J. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986 Jan;18(1):5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Rüschoff J., Bittinger A., Neumann K., Schmitz-Moormann P. Prognostic significance of nucleolar organizing regions (NORs) in carcinomas of the sigmoid colon and rectum. Pathol Res Pract. 1990 Feb;186(1):85–91. doi: 10.1016/S0344-0338(11)81014-9. [DOI] [PubMed] [Google Scholar]
- Strang P., Lindgren A., Stendahl U. Comparison between flow cytometry and single cell cytophotometry for DNA content analysis of the uterine cervix. Acta Radiol Oncol. 1985 Jul-Aug;24(4):337–341. doi: 10.3109/02841868509136062. [DOI] [PubMed] [Google Scholar]
- Suarez V., Newman J., Hiley C., Crocker J., Collins M. The value of NOR numbers in neoplastic and non-neoplastic epithelium of the stomach. Histopathology. 1989 Jan;14(1):61–66. doi: 10.1111/j.1365-2559.1989.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Sugimachi K., Matsuoka H., Ohno S., Mori M., Kuwano H. Multivariate approach for assessing the prognosis of clinical oesophageal carcinoma. Br J Surg. 1988 Nov;75(11):1115–1118. doi: 10.1002/bjs.1800751122. [DOI] [PubMed] [Google Scholar]
- Suresh U. R., Chawner L., Buckley C. H., Fox H. Do AgNOR counts reflect cellular ploidy or cellular proliferation? A study of trophoblastic tissue. J Pathol. 1990 Mar;160(3):213–215. doi: 10.1002/path.1711600306. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Takeuchi T., Nishikawa A., Takami T., Mori H. Nucleolar organizer regions in hepatocarcinogenesis induced by N-2-fluorenylacetamide in rats: comparison with bromodeoxyuridine immunohistochemistry. Jpn J Cancer Res. 1989 Nov;80(11):1047–1051. doi: 10.1111/j.1349-7006.1989.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolley R. C., Schreiber K., Koss L. G., Karas M., Sherman A. DNA distribution in human colon carcinomas and its relationship to clinical behavior. J Natl Cancer Inst. 1982 Jul;69(1):15–22. [PubMed] [Google Scholar]
- Yoshida Y., Okamura T., Kanematsu T., Kakizoe S., Sugimachi K. Comparison between microspectrophotometry and cytofluorometry in measurements of nuclear DNA in human hepatocellular carcinomas. Cancer. 1988 Aug 15;62(4):755–759. doi: 10.1002/1097-0142(19880815)62:4<755::aid-cncr2820620419>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]


