Abstract
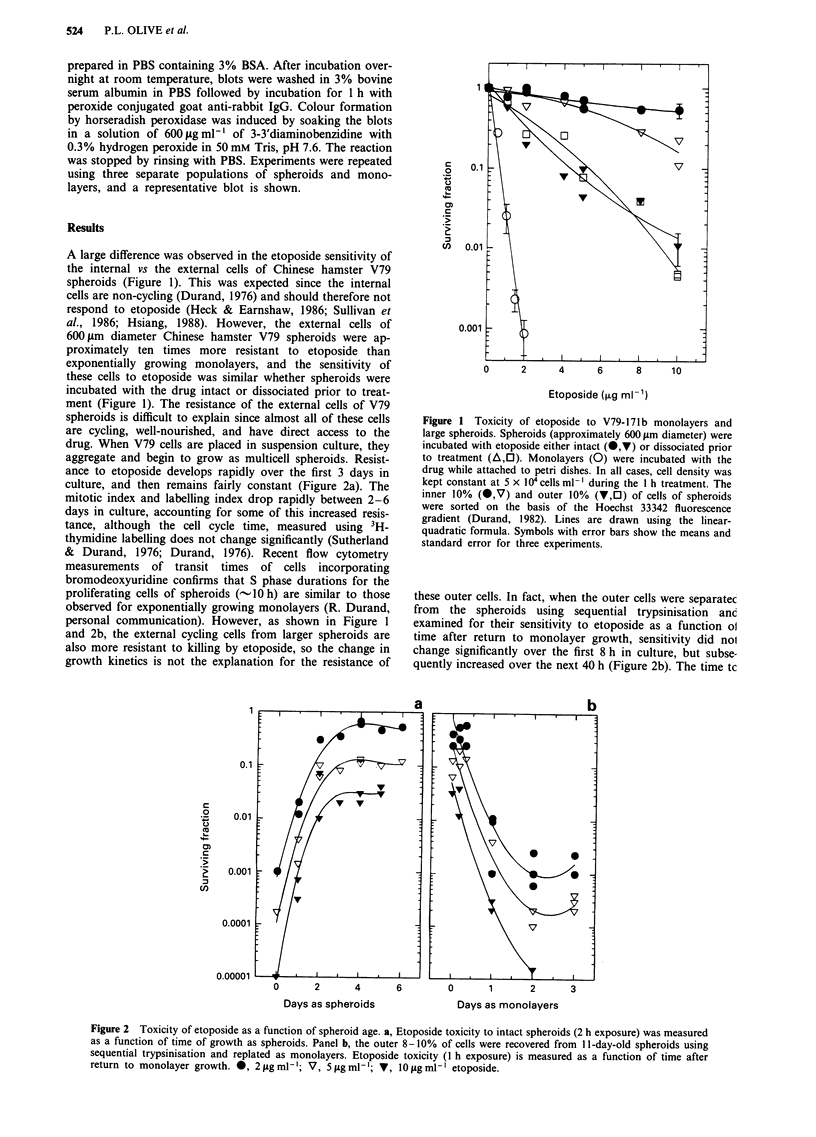
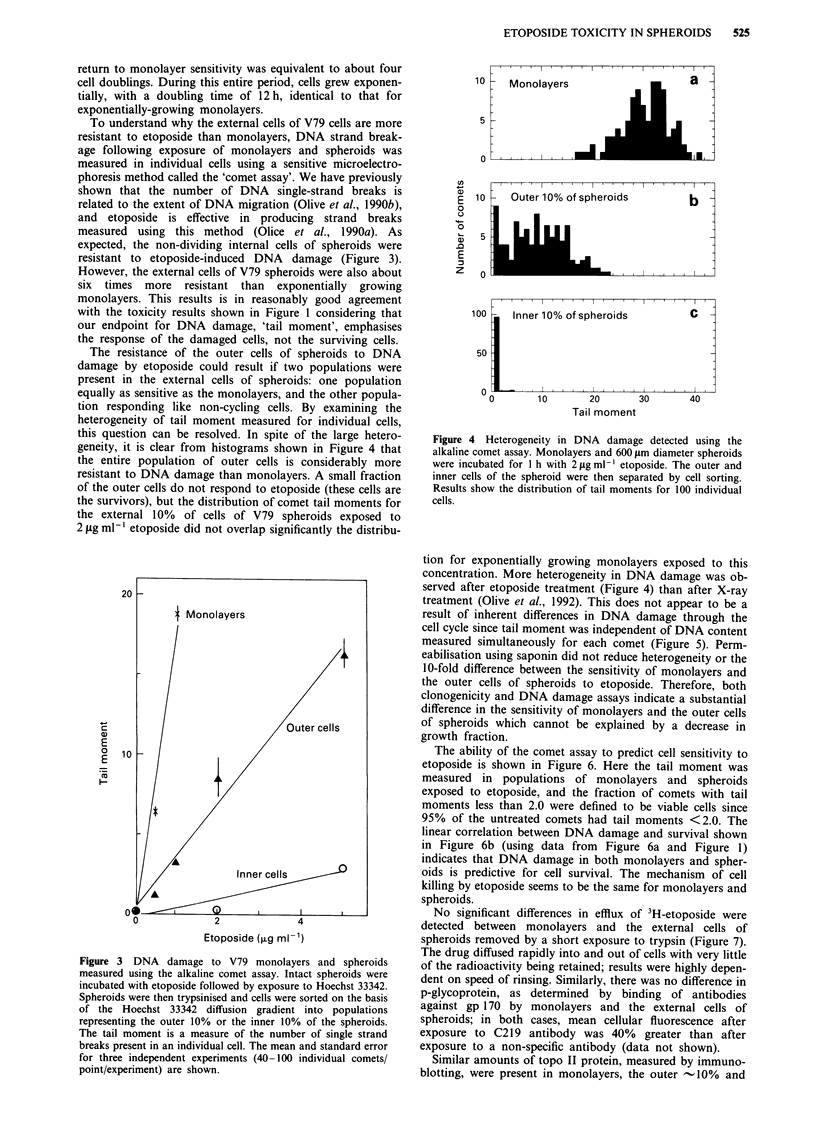
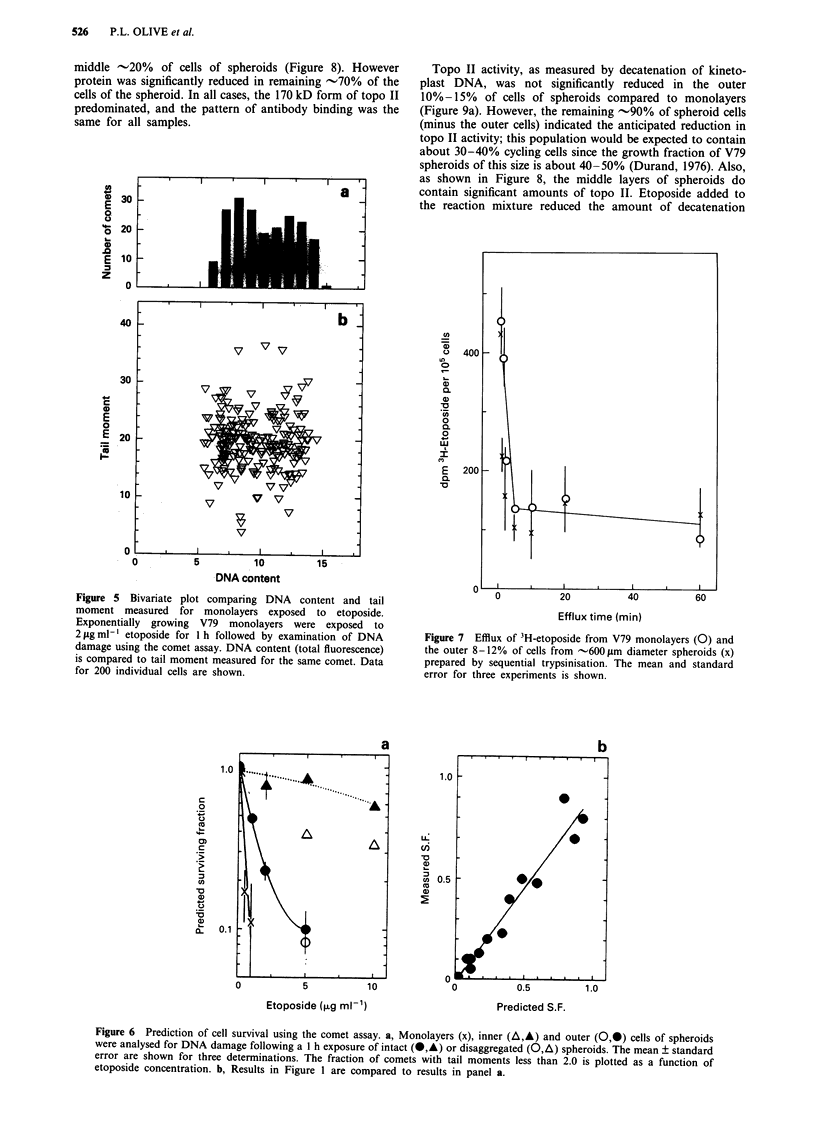
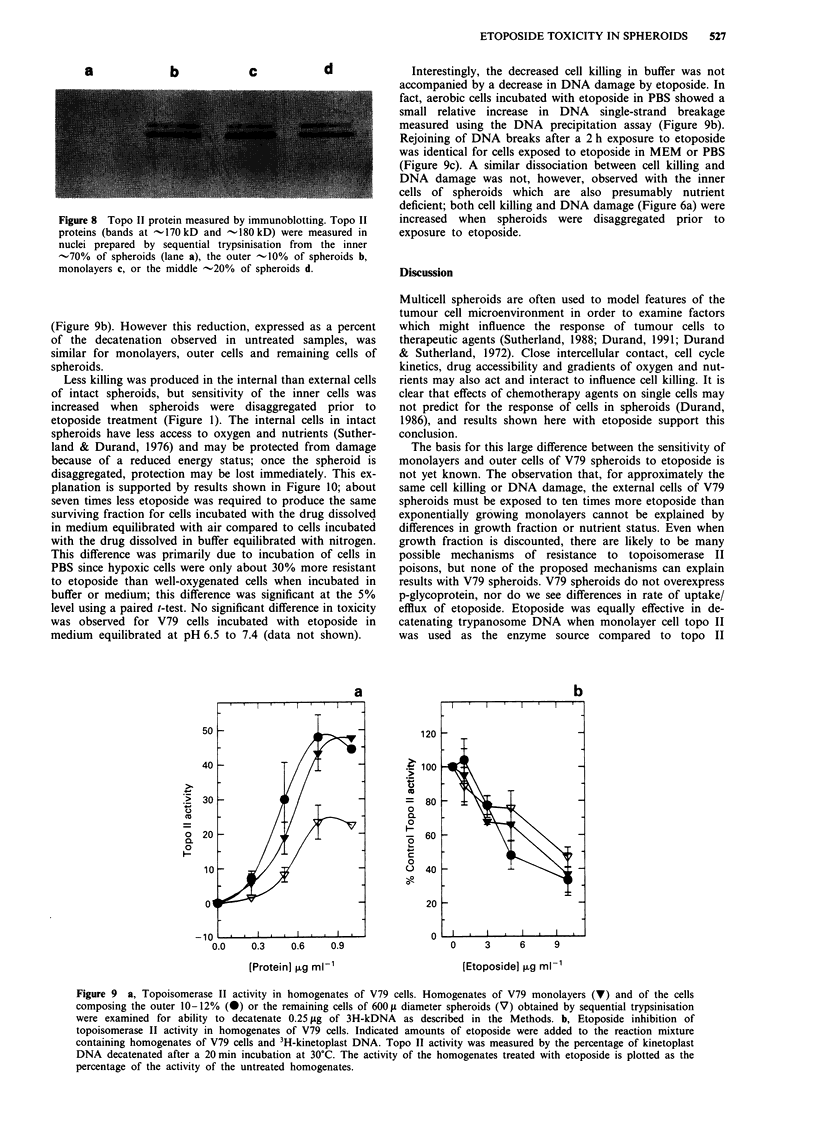
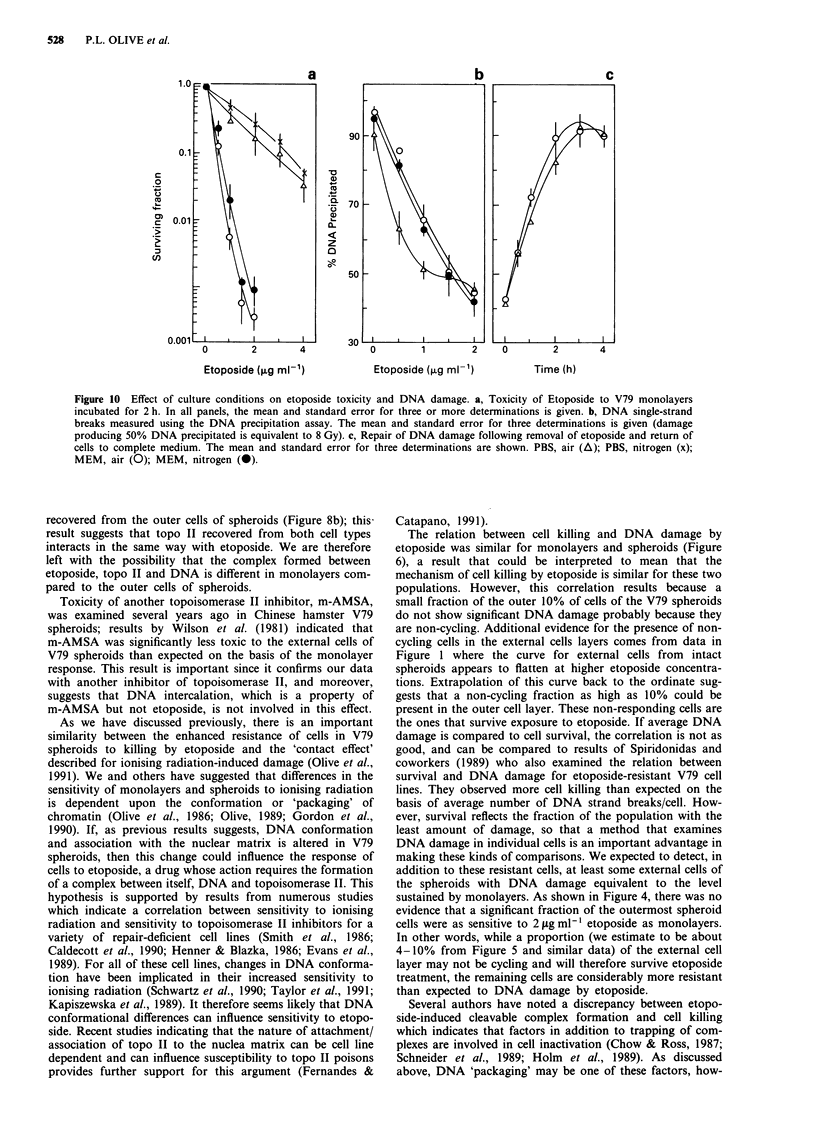
Cells from V79 multicell spheroids must be exposed to approximately 50 times more etoposide than exponentially growing monolayers in order to produce the same amount of cell killing. A part of this difference in sensitivity is readily explained by the decrease in growth fraction of large spheroids, and by the protection afforded by nutrient deprivation which also reduces cellular ATP. However, cells composing the outer 10% of large (approximately 600 microns diameter) V79 spheroids, although actively cycling, were still ten times more resistant to etoposide than exponentially growing monolayers, regardless of whether cells were exposed in situ in spheroids or dispersed by trypsin immediately prior to exposure to the drug. Four cell doublings (48 h) as monolayers were required before the outer cells of spheroids regained drug sensitivity equivalent to that of exponentially growing monolayers. No differences in uptake/efflux of 3H-etoposide or in levels of p-glycoprotein were observed between monolayers and the outer cells of spheroids. In addition, topoisomerase II protein measured by immunoblotting and topoisomerase II activity measured by decatenation of kinetoplast DNA were not reduced in the outer cells of spheroids compared to monolayers. DNA strand breakage measured in individual cells using the DNA precipitation and comet assays correlated well with cell killing with one exception: DNA damage was not affected when cells were incubated with etoposide in phosphate-buffered saline, although the etoposide concentration required to produce a given amount of cell killing was increased approximately 7-fold compared to cells incubated with the drug in complete medium. These results indicate that etoposide toxicity towards V79 spheroids is influenced not only by proliferative status of the cells but also by factors which may include DNA packaging and the growth environment of the cell prior to and during treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldecott K., Banks G., Jeggo P. DNA double-strand break repair pathways and cellular tolerance to inhibitors of topoisomerase II. Cancer Res. 1990 Sep 15;50(18):5778–5783. [PubMed] [Google Scholar]
- Chow K. C., Ross W. E. Topoisomerase-specific drug sensitivity in relation to cell cycle progression. Mol Cell Biol. 1987 Sep;7(9):3119–3123. doi: 10.1128/mcb.7.9.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arpa P., Beardmore C., Liu L. F. Involvement of nucleic acid synthesis in cell killing mechanisms of topoisomerase poisons. Cancer Res. 1990 Nov 1;50(21):6919–6924. [PubMed] [Google Scholar]
- D'Arpa P., Liu L. F. Topoisomerase-targeting antitumor drugs. Biochim Biophys Acta. 1989 Dec 17;989(2):163–177. doi: 10.1016/0304-419x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Danks M. K., Schmidt C. A., Cirtain M. C., Suttle D. P., Beck W. T. Altered catalytic activity of and DNA cleavage by DNA topoisomerase II from human leukemic cells selected for resistance to VM-26. Biochemistry. 1988 Nov 29;27(24):8861–8869. doi: 10.1021/bi00424a026. [DOI] [PubMed] [Google Scholar]
- Durand R. E. Cell cycle kinetics in an in vitro tumor model. Cell Tissue Kinet. 1976 Sep;9(5):403–412. doi: 10.1111/j.1365-2184.1976.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Durand R. E. Chemosensitivity testing in V79 spheroids: drug delivery and cellular microenvironment. J Natl Cancer Inst. 1986 Jul;77(1):247–252. [PubMed] [Google Scholar]
- Durand R. E., Sutherland R. M. Effects of intercellular contact on repair of radiation damage. Exp Cell Res. 1972 Mar;71(1):75–80. doi: 10.1016/0014-4827(72)90265-0. [DOI] [PubMed] [Google Scholar]
- Durand R. E. Use of Hoechst 33342 for cell selection from multicell systems. J Histochem Cytochem. 1982 Feb;30(2):117–122. doi: 10.1177/30.2.6174559. [DOI] [PubMed] [Google Scholar]
- Evans H. H., Ricanati M., Horng M. F., Mencl J. Relationship between topoisomerase II and radiosensitivity in mouse L5178Y lymphoma strains. Mutat Res. 1989 Jan;217(1):53–63. doi: 10.1016/0921-8777(89)90036-0. [DOI] [PubMed] [Google Scholar]
- Fernandes D. J., Catapano C. V. Nuclear matrix targets for anticancer agents. Cancer Cells. 1991 Apr;3(4):134–140. [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Gordon D. J., Milner A. E., Beaney R. P., Grdina D. J., Vaughan A. T. The increase in radioresistance of Chinese hamster cells cultured as spheroids is correlated to changes in nuclear morphology. Radiat Res. 1990 Feb;121(2):175–179. [PubMed] [Google Scholar]
- Haim N., Nemec J., Roman J., Sinha B. K. Peroxidase-catalyzed metabolism of etoposide (VP-16-213) and covalent binding of reactive intermediates to cellular macromolecules. Cancer Res. 1987 Nov 15;47(22):5835–5840. [PubMed] [Google Scholar]
- Heck M. M., Earnshaw W. C. Topoisomerase II: A specific marker for cell proliferation. J Cell Biol. 1986 Dec;103(6 Pt 2):2569–2581. doi: 10.1083/jcb.103.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Blazka M. E. Hypersensitivity of cultured ataxia-telangiectasia cells to etoposide. J Natl Cancer Inst. 1986 Jun;76(6):1007–1011. [PubMed] [Google Scholar]
- Holm C., Covey J. M., Kerrigan D., Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989 Nov 15;49(22):6365–6368. [PubMed] [Google Scholar]
- Hsiang Y. H., Wu H. Y., Liu L. F. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988 Jun 1;48(11):3230–3235. [PubMed] [Google Scholar]
- Kalwinsky D. K., Look A. T., Ducore J., Fridland A. Effects of the epipodophyllotoxin VP-16-213 on cell cycle traverse, DNA synthesis, and DNA strand size in cultures of human leukemic lymphoblasts. Cancer Res. 1983 Apr;43(4):1592–1597. [PubMed] [Google Scholar]
- Kapiszewska M., Wright W. D., Lange C. S., Roti Roti J. L. DNA supercoiling changes in nucleoids from irradiated L5178Y-S and -R cells. Radiat Res. 1989 Sep;119(3):569–575. [PubMed] [Google Scholar]
- Kasahara K., Fujiwara Y., Sugimoto Y., Nishio K., Tamura T., Matsuda T., Saijo N. Determinants of response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst. 1992 Jan 15;84(2):113–118. doi: 10.1093/jnci/84.2.113. [DOI] [PubMed] [Google Scholar]
- Kupfer G., Bodley A. L., Liu L. F. Involvement of intracellular ATP in cytotoxicity of topoisomerase II-targetting antitumor drugs. NCI Monogr. 1987;(4):37–40. [PubMed] [Google Scholar]
- Olive P. L., Banáth J. P., Durand R. E. Detection of etoposide resistance by measuring DNA damage in individual Chinese hamster cells. J Natl Cancer Inst. 1990 May 2;82(9):779–783. doi: 10.1093/jnci/82.9.779. [DOI] [PubMed] [Google Scholar]
- Olive P. L. Cell proliferation as a requirement for development of the contact effect in Chinese hamster V79 spheroids. Radiat Res. 1989 Jan;117(1):79–92. [PubMed] [Google Scholar]
- Olive P. L. DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen. 1988;11(4):487–495. doi: 10.1002/em.2850110409. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Durand R. E., Banáth J. P., Evans H. H. Etoposide sensitivity and topoisomerase II activity in Chinese hamster V79 monolayers and small spheroids. Int J Radiat Biol. 1991 Sep;60(3):453–466. doi: 10.1080/09553009114552311. [DOI] [PubMed] [Google Scholar]
- Olive P. L. Fluorescent probes for cellular hypoxia: lack of transfer of fluorescence between cells in vitro. Int J Radiat Oncol Biol Phys. 1985 Nov;11(11):1947–1954. doi: 10.1016/0360-3016(85)90276-7. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Hilton J., Durand R. E. DNA conformation of Chinese hamster V79 cells and sensitivity to ionizing radiation. Radiat Res. 1986 Jul;107(1):115–124. [PubMed] [Google Scholar]
- Olive P. L., MacPhail S. H. Radiation-induced DNA unwinding is influenced by cell shape and trypsin. Radiat Res. 1992 May;130(2):241–248. [PubMed] [Google Scholar]
- Olive P. L., Wlodek D., Durand R. E., Banáth J. P. Factors influencing DNA migration from individual cells subjected to gel electrophoresis. Exp Cell Res. 1992 Feb;198(2):259–267. doi: 10.1016/0014-4827(92)90378-l. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Biochemical basis for the interactions of type I and type II topoisomerases with DNA. Pharmacol Ther. 1989;41(1-2):223–241. doi: 10.1016/0163-7258(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Ostling O., Johanson K. J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984 Aug 30;123(1):291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- Sahai B. M., Kaplan J. G. A quantitative decatenation assay for type II topoisomerases. Anal Biochem. 1986 Aug 1;156(2):364–379. doi: 10.1016/0003-2697(86)90267-8. [DOI] [PubMed] [Google Scholar]
- Schneider E., Lawson P. A., Ralph R. K. Inhibition of protein synthesis reduces the cytotoxicity of 4'-(9-acridinylamino)methanesulfon-m-anisidide without affecting DNA breakage and DNA topoisomerase II in a murine mastocytoma cell line. Biochem Pharmacol. 1989 Jan 15;38(2):263–269. doi: 10.1016/0006-2952(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Schwartz J. L., Shadley J. D., Jaffe D. R., Whitlock J., Rotmensch J., Cowan J. M., Gordon D. J., Vaughan A. T. Association between radiation sensitivity, DNA repair and chromosome organization in the Chinese hamster ovary cell line xrs-5. Prog Clin Biol Res. 1990;340A:255–264. [PubMed] [Google Scholar]
- Smith P. J., Anderson C. O., Watson J. V. Predominant role for DNA damage in etoposide-induced cytotoxicity and cell cycle perturbation in human SV40-transformed fibroblasts. Cancer Res. 1986 Nov;46(11):5641–5645. [PubMed] [Google Scholar]
- Spiridonidis C. A., Chatterjee S., Petzold S. J., Berger N. A. Topoisomerase II-dependent and -independent mechanisms of etoposide resistance in Chinese hamster cell lines. Cancer Res. 1989 Feb 1;49(3):644–650. [PubMed] [Google Scholar]
- Sullivan D. M., Glisson B. S., Hodges P. K., Smallwood-Kentro S., Ross W. E. Proliferation dependence of topoisomerase II mediated drug action. Biochemistry. 1986 Apr 22;25(8):2248–2256. doi: 10.1021/bi00356a060. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Durand R. E. Radiation response of multicell spheroids--an in vitro tumour model. Curr Top Radiat Res Q. 1976 Jan;11(1):87–139. [PubMed] [Google Scholar]
- Taylor Y. C., Duncan P. G., Zhang X., Wright W. D. Differences in the DNA supercoiling response of irradiated cell lines from ataxia-telangiectasia versus unaffected individuals. Int J Radiat Biol. 1991 Feb;59(2):359–371. doi: 10.1080/09553009114550331. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Whitmore G. F., Hill R. P. Activity of 4'-(9-acridinylamino)methanesulfon-m-anisidide against Chinese hamster cells in multicellular spheroids. Cancer Res. 1981 Jul;41(7):2817–2822. [PubMed] [Google Scholar]



