Abstract
Chemical structures of the sugar chains of various human alpha-fetoprotein (AFP) species with different affinity for Concanavalin A (Con A) and Lens culinaris agglutinin (LCA) were examined by pyridylamination of their oligosaccharides and stepwise exoglycosidase digestion. Using reversed-phase and size-fractionation high performance liquid chromatography systems we identified six pyridylamino-sugar chains. The Con A-reactive and LCA-nonreactive species of AFP from patients with hepatocellular carcinoma contained a biantennary sugar chain, and the Con A-reactive and LCA-reactive species had a biantennary one with a fucose residue at the innermost N-acetylglucosamine residue. The Con A-nonreactive and LCA-reactive species contained a biantennary sugar chain both with a bisecting-N-acetylglucosamine residue at the trimannosyl core and with a focus residue at the innermost N-acetylglucosamine residue. The Con A-nonreactive and LCA-nonreactive species contained a fucosylated triantennary sugar chain as a major component, and two minor components: a triantennary sugar chain and a biantennary sugar chain with a bisecting-N-acetylglucosamine residue at the trimannosyl core. Thus, the fucosylated and non-fucosylated triantennary sugar chains were newly identified in human AFP. Essentially identical results were obtained for AFP from the patient with gallbladder carcinoma which metastasizes to the liver. These results indicate that the increment in fucosylation and branching to form new antennae is a characteristic feature of the carbohydrate chains of AFP from patients with neoplastic diseases of the liver.
Full text
PDF

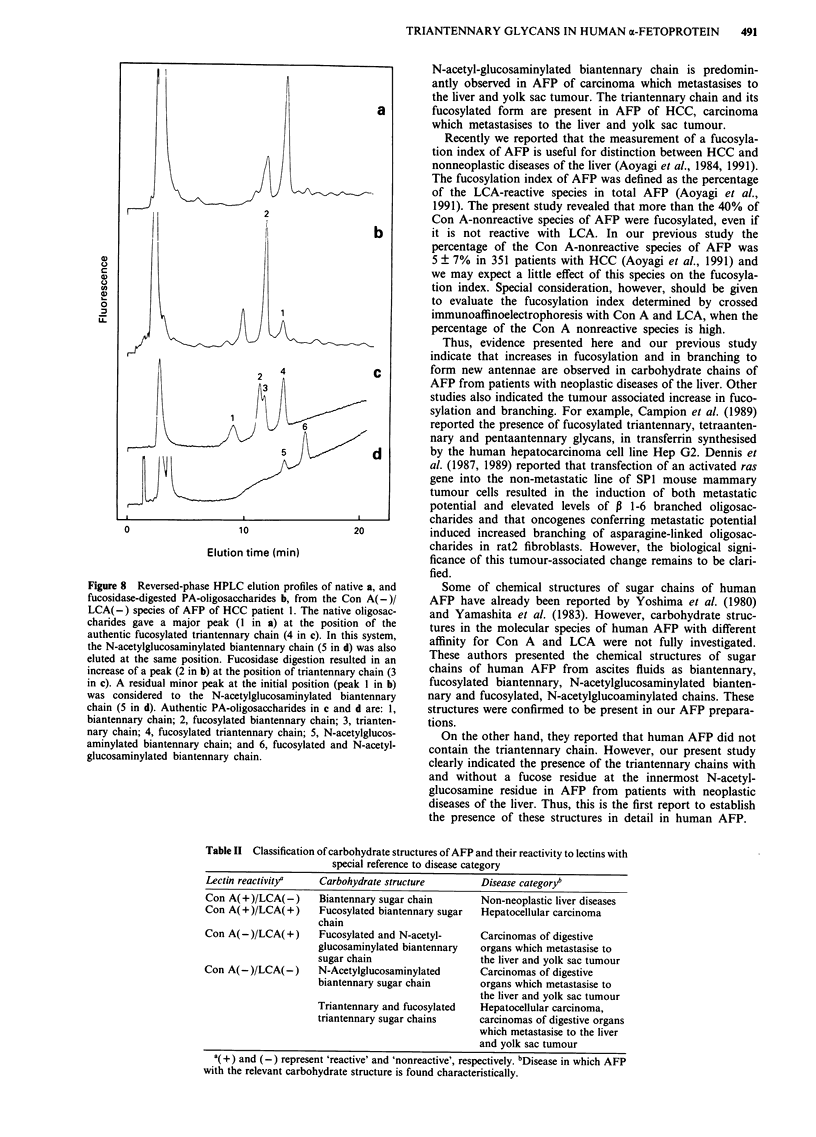

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Production of embryonal serum alpha-globulin by hepatomas: review of experimental and clinical data. Cancer Res. 1968 Jul;28(7):1344–1350. [PubMed] [Google Scholar]
- Alpert E., Feller E. R. Alpha-fetoprotein (AFP) in benign liver disease. Evidence that normal liver regeneration does not induce AFP synthesis. Gastroenterology. 1978 May;74(5 Pt 1):856–858. [PubMed] [Google Scholar]
- Alpert E., Pinn V. W., Isselbacher K. J. Alpha-fetoprotein in a patient with gastric carcinoma metastatic to the liver. N Engl J Med. 1971 Nov 4;285(19):1058–1059. doi: 10.1056/NEJM197111042851905. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y., Ikenaka T., Ichida F. Comparative chemical structures of human alpha-fetoproteins from fetal serum and from ascites fluid of a patient with hepatoma. Cancer Res. 1977 Oct;37(10):3663–3667. [PubMed] [Google Scholar]
- Aoyagi Y., Isemura M., Yosizawa Z., Suzuki Y., Sekine C., Ono T., Ichida F. Fucosylation of serum alpha-fetoprotein in patients with primary hepatocellular carcinoma. Biochim Biophys Acta. 1985 Aug 23;830(3):217–223. doi: 10.1016/0167-4838(85)90277-8. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y., Suzuki Y., Igarashi K., Saitoh A., Oguro M., Yokota T., Mori S., Nomoto M., Isemura M., Asakura H. The usefulness of simultaneous determinations of glucosaminylation and fucosylation indices of alpha-fetoprotein in the differential diagnosis of neoplastic diseases of the liver. Cancer. 1991 May 1;67(9):2390–2394. doi: 10.1002/1097-0142(19910501)67:9<2390::aid-cncr2820670928>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y., Suzuki Y., Isemura M., Soga K., Ozaki T., Ichida T., Inoue K., Sasaki H., Ichida F. Differential reactivity of alpha-fetoprotein with lectins and evaluation of its usefulness in the diagnosis of hepatocellular carcinoma. Gan. 1984 Sep;75(9):809–815. [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Bayard B., Kerckaert J. P., Strecker G., Dorland L., van Halbeek H., Vliegenthart J. F. Structure determination of the carbohydrate chains of rat alpha-fetoprotein. Eur J Biochem. 1983 Dec 1;137(1-2):319–323. doi: 10.1111/j.1432-1033.1983.tb07831.x. [DOI] [PubMed] [Google Scholar]
- Breborowicz J., Mackiewicz A., Breborowicz D. Microheterogeneity of alpha-fetoprotein in patient serum as demonstrated by lectin affino-electrophoresis. Scand J Immunol. 1981 Jul;14(1):15–20. doi: 10.1111/j.1365-3083.1981.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Campion B., Léger D., Wieruszeski J. M., Montreuil J., Spik G. Presence of fucosylated triantennary, tetraantennary and pentaantennary glycans in transferrin synthesized by the human hepatocarcinoma cell line Hep G2. Eur J Biochem. 1989 Sep 15;184(2):405–413. doi: 10.1111/j.1432-1033.1989.tb15032.x. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Kosh K., Bryce D. M., Breitman M. L. Oncogenes conferring metastatic potential induce increased branching of Asn-linked oligosaccharides in rat2 fibroblasts. Oncogene. 1989 Jul;4(7):853–860. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Hase S., Ibuki T., Ikenaka T. Reexamination of the pyridylamination used for fluorescence labeling of oligosaccharides and its application to glycoproteins. J Biochem. 1984 Jan;95(1):197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- Ishiguro T., Sugitachi I., Sakaguchi H., Itani S. Serum alpha-fetoprotein subfractions in patients with primary hepatoma or hepatic metastasis of gastric cancer. Cancer. 1985 Jan 1;55(1):156–159. doi: 10.1002/1097-0142(19850101)55:1<156::aid-cncr2820550124>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Karvountzis G. G., Redeker A. G. Relation of alpha-fetoprotein in acute hepatitis to severity and prognosis. Ann Intern Med. 1974 Feb;80(2):156–160. doi: 10.7326/0003-4819-80-2-156. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Carbohydrate structure of the concanavalin A molecular variants of alpha-fetoprotein. J Biol Chem. 1982 Apr 10;257(7):3453–3457. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIntire K. R., Waldmann T. A., Moertel C. G., Go V. L. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975 Apr;35(4):991–996. [PubMed] [Google Scholar]
- Nishiura T., Fujii S., Kanayama Y., Nishikawa A., Tomiyama Y., Iida M., Karasuno T., Nakao H., Yonezawa T., Taniguchi N. Carbohydrate analysis of immunoglobulin G myeloma proteins by lectin and high performance liquid chromatography: role of glycosyltransferases in the structures. Cancer Res. 1990 Sep 1;50(17):5345–5350. [PubMed] [Google Scholar]
- O'Conor G. T., Tatarinov Y. S., Abelev G. I., Uriel J. A collaborative study for the evaluation of a serologic test for primary liver cancer. Cancer. 1970 May;25(5):1091–1098. doi: 10.1002/1097-0142(197005)25:5<1091::aid-cncr2820250514>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Pekkala A., Seppälä M. Developmental changes in carbohydrate moiety of human alpha-fetoprotein. Int J Cancer. 1978 Nov 15;22(5):515–520. doi: 10.1002/ijc.2910220502. [DOI] [PubMed] [Google Scholar]
- Taketa K., Hirai H. Lectin affinity electrophoresis of alpha-fetoprotein in cancer diagnosis. Electrophoresis. 1989 Aug-Sep;10(8-9):562–567. doi: 10.1002/elps.1150100805. [DOI] [PubMed] [Google Scholar]
- Tomiya N., Awaya J., Kurono M., Endo S., Arata Y., Takahashi N. Analyses of N-linked oligosaccharides using a two-dimensional mapping technique. Anal Biochem. 1988 May 15;171(1):73–90. doi: 10.1016/0003-2697(88)90126-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Hase S., Fukuda S., Sano O., Ikenaka T. Structures of the sugar chains of interferon-gamma produced by human myelomonocyte cell line HBL-38. J Biochem. 1989 Apr;105(4):547–555. doi: 10.1093/oxfordjournals.jbchem.a122703. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Tsuchida Y., Nishi S., Kobata A. Sugar chain of alpha-fetoprotein produced in human yolk sac tumor. Cancer Res. 1983 Oct;43(10):4691–4695. [PubMed] [Google Scholar]
- Yoshima H., Mizuochi T., Ishii M., Kobata A. Structure of the asparagine-linked sugar chains of alpha-fetoprotein purified from human ascites fluid. Cancer Res. 1980 Nov;40(11):4276–4281. [PubMed] [Google Scholar]