Abstract
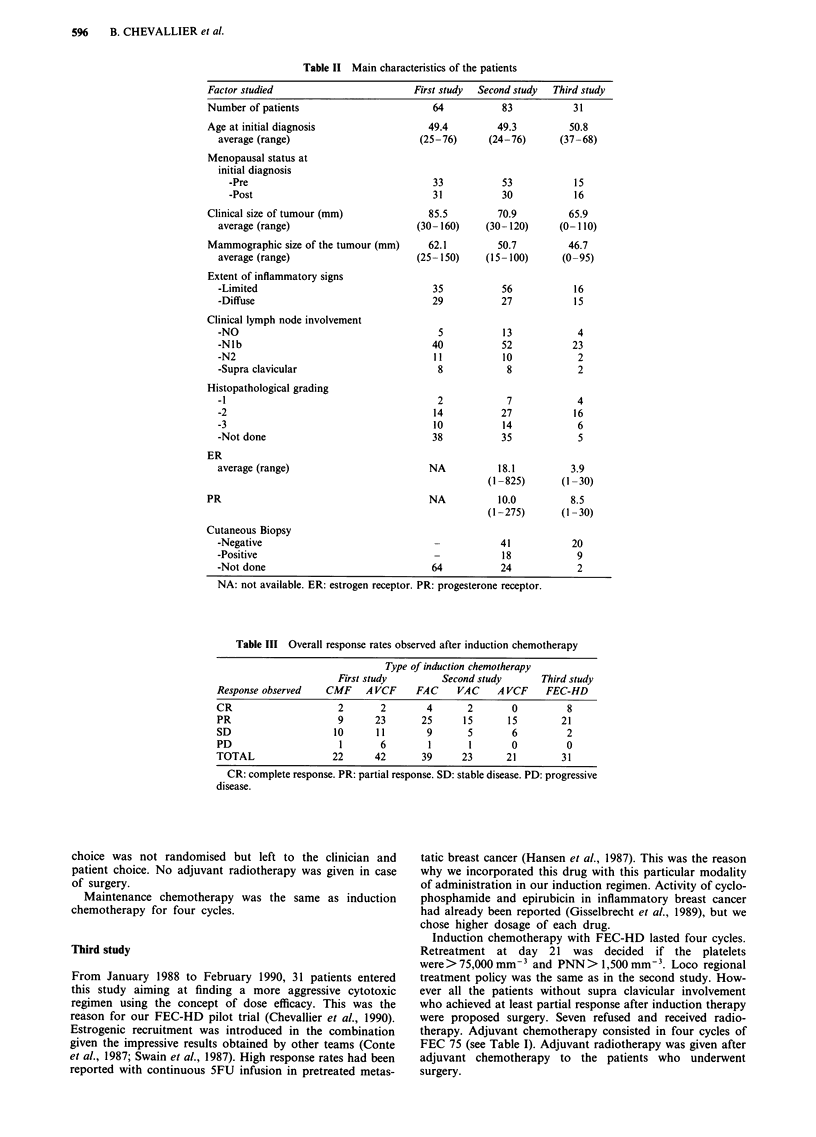
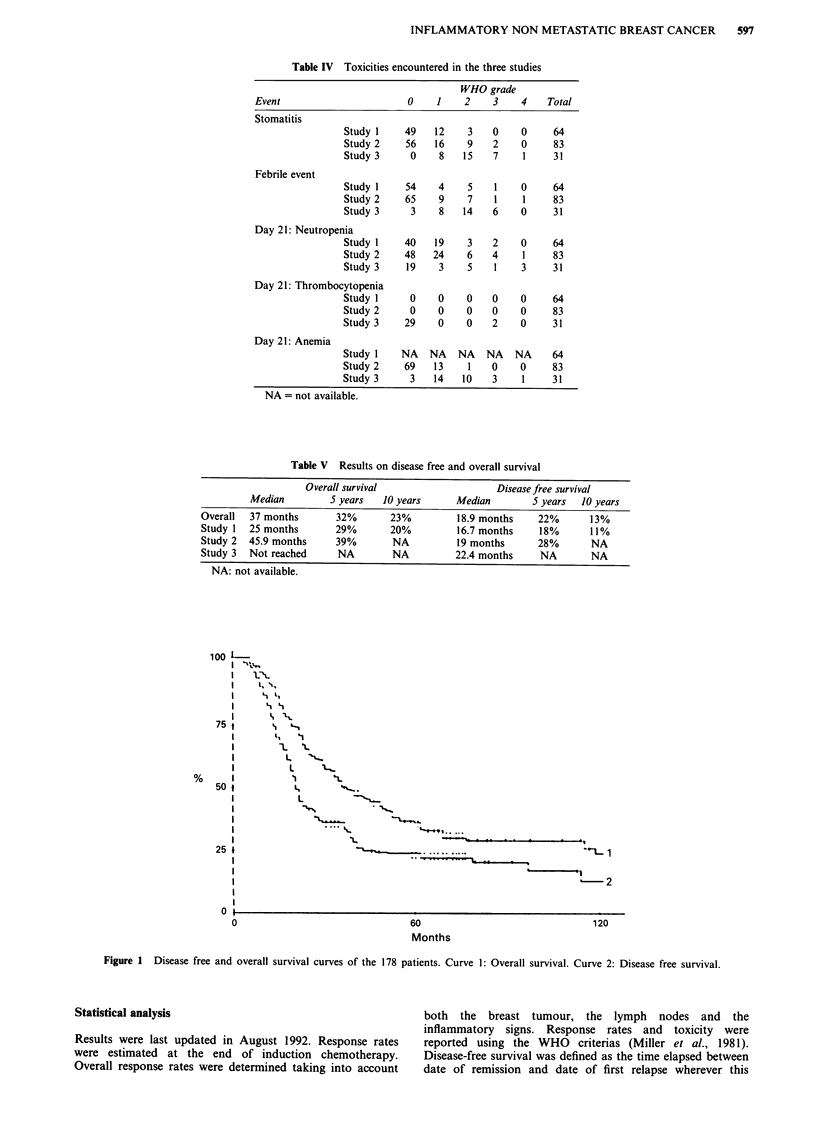
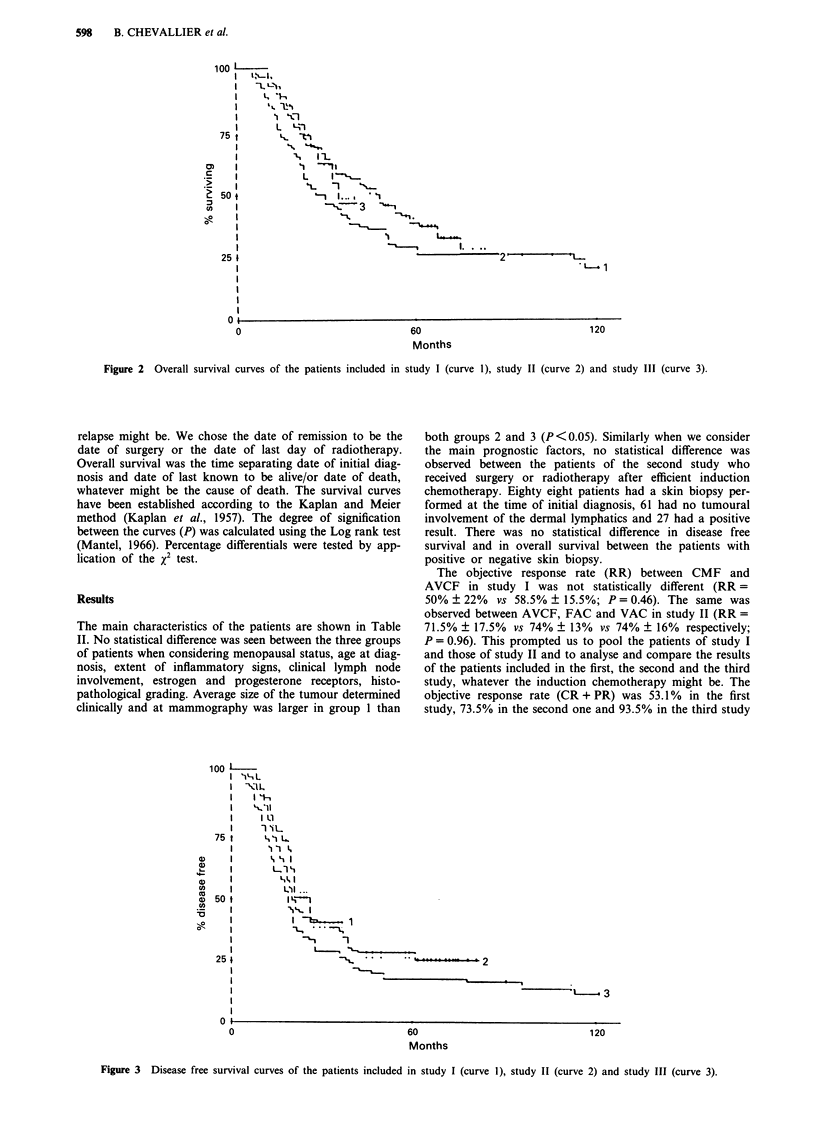
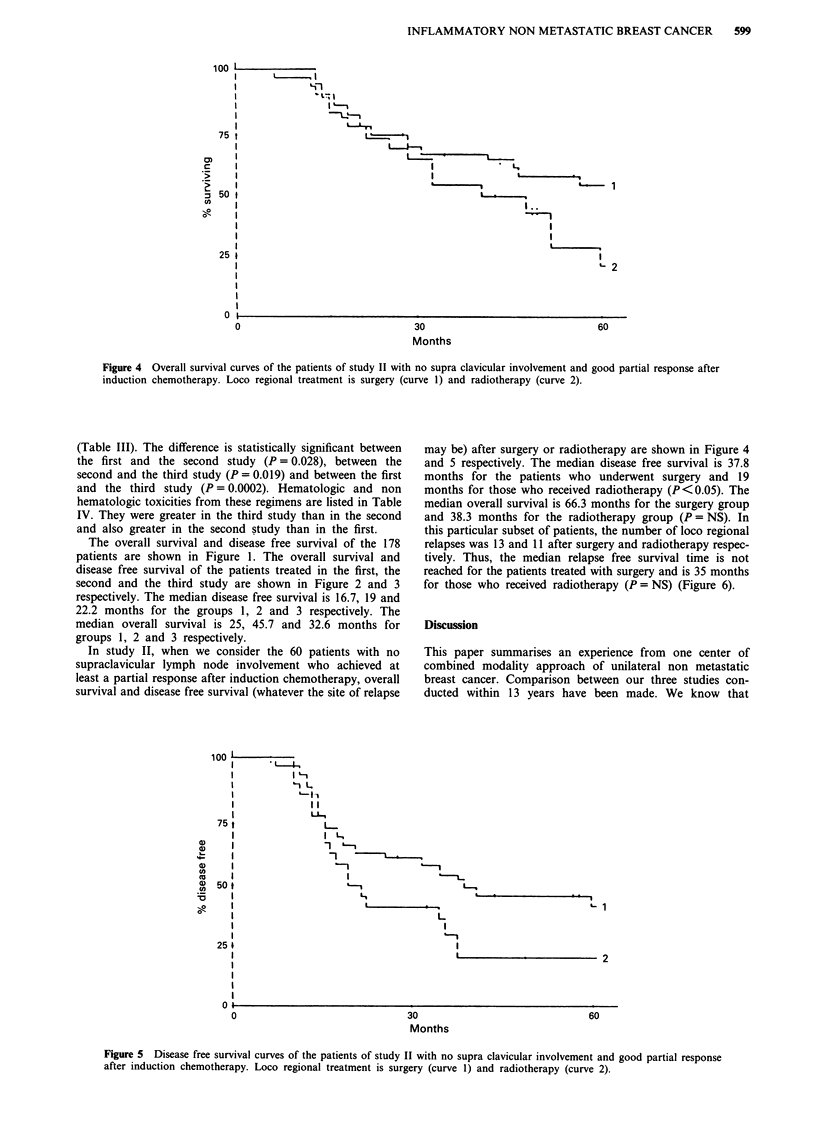
One hundred and seventy-eight patients with non metastatic inflammatory breast cancer (IBC) have been treated at the Centre H. Becquerel. Median follow up is 67 months (6-178). Every patient received neoadjuvant chemotherapy (mean number of cycles = 4; range: 2-8), followed by a loco regional treatment (radiotherapy = XRT or modified radical mastectomy = S), followed by adjuvant chemotherapy. During this period, the types of chemotherapy and locoregional treatment have been the following: Study I: 64 patients treated with CMF or AVCF and XRT; Study II: 83 patients, treated with either AVCF, FAC or VAC followed by S (n = 38) or XRT (n = 22) in case of complete or partial response, or followed by XRT (23) in case of initial supraclavicular lymph node involvement or lack of response after chemotherapy; Study III: 31 patients treated with FEC-HD + Estrogenic recruitment followed by S and XRT after adjuvant chemotherapy, except seven patients who received XRT (refusal of surgery). Although objective response rates (= 56.2, 73.5 and 93.5% for study I, II and III respectively) are statistically better in the 3rd study, this does not translate in dramatically different disease free survival (median = 16.7, 19 and 22.2 months respectively for study I, II and III) or overall survival (median = 25, 45.7 and 32.6 months respectively for study I, II and III). Analysis of subset of patients without supra clavicular lymph node involvement where neoadjuvant chemotherapy obtained at least a 50% response reveals a median disease free survival and median overall survival of respectively 38.3 and 60.1 months for patients who underwent S vs 19 and 38.3 months for those who received XRT (P = 0.15). These studies suggest that surgery has no deleterious effect on outcome of IBC. Advantage on disease free survival or overall survival from intensive chemotherapy in IBC remains to be proven with appropriate randomised trials.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calderoli H., de Manzini N., Keiling R. Role of chemotherapy in acute breast cancer. Analysis of 41 cases. Int Surg. 1988 Apr-Jun;73(2):112–115. [PubMed] [Google Scholar]
- Camp E., 3rd Inflammatory carcinoma of the breast: the case for conservatism. Am J Surg. 1976 May;131(5):583–586. doi: 10.1016/0002-9610(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Chevallier B., Asselain B., Kunlin A., Veyret C., Bastit P., Graic Y. Inflammatory breast cancer. Determination of prognostic factors by univariate and multivariate analysis. Cancer. 1987 Aug 15;60(4):897–902. doi: 10.1002/1097-0142(19870815)60:4<897::aid-cncr2820600430>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Conte P. F., Alama A., Bertelli G., Canavese G., Carnino F., Catturich A., Di Marco E., Gardin G., Jacomuzzi A., Monzeglio C. Chemotherapy with estrogenic recruitment and surgery in locally advanced breast cancer: clinical and cytokinetic results. Int J Cancer. 1987 Oct 15;40(4):490–494. doi: 10.1002/ijc.2910400410. [DOI] [PubMed] [Google Scholar]
- Davidson N. E., Lippman M. E. Stimulation of breast cancer with estrogens: how much clinical value? Eur J Cancer Clin Oncol. 1987 Jul;23(7):897–900. doi: 10.1016/0277-5379(87)90332-4. [DOI] [PubMed] [Google Scholar]
- Fastenberg N. A., Martin R. G., Buzdar A. U., Hortobagyi G. N., Montague E. D., Blumenschein G. R., Jessup J. M. Management of inflammatory carcinoma of the breast. A combined modality approach. Am J Clin Oncol. 1985 Apr;8(2):134–141. doi: 10.1097/00000421-198504000-00005. [DOI] [PubMed] [Google Scholar]
- Feldman L. D., Hortobagyi G. N., Buzdar A. U., Ames F. C., Blumenschein G. R. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res. 1986 May;46(5):2578–2581. [PubMed] [Google Scholar]
- Fields J. N., Kuske R. R., Perez C. A., Fineberg B. B., Bartlett N. Prognostic factors in inflammatory breast cancer. Univariate and multivariate analysis. Cancer. 1989 Mar 15;63(6):1225–1232. doi: 10.1002/1097-0142(19890315)63:6<1225::aid-cncr2820630632>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht C., Lepage E., Extra J. M., Espie M., Andolenko P., Morvan F., Ganem G., Gerota Y., Bourstyn E., Marty M. Cancer du sein: traitement intensif avec autogreffe de moelle. Bull Cancer. 1989;76(1):99–104. [PubMed] [Google Scholar]
- Hagelberg R. S., Jolly P. C., Anderson R. P. Role of surgery in the treatment of inflammatory breast carcinoma. Am J Surg. 1984 Jul;148(1):125–131. doi: 10.1016/0002-9610(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Hansen R., Quebbeman E., Beatty P., Ritch P., Anderson T., Jenkins D., Frick J., Ausman R. Continuous 5-fluorouracil infusion in refractory carcinoma of the breast. Breast Cancer Res Treat. 1987 Nov;10(2):145–149. doi: 10.1007/BF01810577. [DOI] [PubMed] [Google Scholar]
- Hortobagyi G. N., Buzdar A. U. Progress in inflammatory breast cancer: cause for cautious optimism. J Clin Oncol. 1986 Dec;4(12):1727–1729. doi: 10.1200/JCO.1986.4.12.1727. [DOI] [PubMed] [Google Scholar]
- Hryniuk W. M., Figueredo A., Goodyear M. Applications of dose intensity to problems in chemotherapy of breast and colorectal cancer. Semin Oncol. 1987 Dec;14(4 Suppl 4):3–11. [PubMed] [Google Scholar]
- Israël L., Breau J. L., Morere J. F. Two years of high-dose cyclophosphamide and 5-fluorouracil followed by surgery after 3 months for acute inflammatory breast carcinomas. A phase II study of 25 cases with a median follow-up of 35 months. Cancer. 1986 Jan 1;57(1):24–28. doi: 10.1002/1097-0142(19860101)57:1<24::aid-cncr2820570107>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Klasa R. J., Murray N., Coldman A. J. Dose-intensity meta-analysis of chemotherapy regimens in small-cell carcinoma of the lung. J Clin Oncol. 1991 Mar;9(3):499–508. doi: 10.1200/JCO.1991.9.3.499. [DOI] [PubMed] [Google Scholar]
- Lucas F. V., Perez-Mesa C. Inflammatory carcinoma of the breast. Cancer. 1978 Apr;41(4):1595–1605. doi: 10.1002/1097-0142(197804)41:4<1595::aid-cncr2820410450>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Maloisel F., Dufour P., Bergerat J. P., Herbrecht R., Duclos B., Boilletot A., Giron C., Jaeck D., Haennel P., Jung G. Results of initial doxorubicin, 5-fluorouracil, and cyclophosphamide combination chemotherapy for inflammatory carcinoma of the breast. Cancer. 1990 Feb 15;65(4):851–855. doi: 10.1002/1097-0142(19900215)65:4<851::aid-cncr2820650403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mourali N., Tabbane F., Muenz L. R., Bahi J., Belhassen S., Kamaraju L. S., Levine P. H. Preliminary results of primary systemic chemotherapy in association with surgery or radiotherapy in rapidly progressing breast cancer. Br J Cancer. 1982 Mar;45(3):367–374. doi: 10.1038/bjc.1982.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S., Miyauchi K., Nishizawa Y., Koyama H., Terasawa T. Management of inflammatory carcinoma of the breast with combined modality therapy including intraarterial infusion chemotherapy as an induction therapy. Long-term follow-up results of 28 patients. Cancer. 1988 Apr 15;61(8):1483–1491. doi: 10.1002/1097-0142(19880415)61:8<1483::aid-cncr2820610802>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rouëssé J., Friedman S., Mouriesse H., Sarrazin D., Spielmann M. Therapeutic strategies in inflammatory breast carcinoma based on prognostic factors. Breast Cancer Res Treat. 1990 Jul;16(1):15–22. doi: 10.1007/BF01806571. [DOI] [PubMed] [Google Scholar]
- Rouëssé J., Friedman S., Sarrazin D., Mouriesse H., Le Chevalier T., Arriagada R., Spielmann M., Papacharalambous A., May-Levin F. Primary chemotherapy in the treatment of inflammatory breast carcinoma: a study of 230 cases from the Institut Gustave-Roussy. J Clin Oncol. 1986 Dec;4(12):1765–1771. doi: 10.1200/JCO.1986.4.12.1765. [DOI] [PubMed] [Google Scholar]
- Schäfer P., Alberto P., Forni M., Obradovic D., Pipard G., Krauer F. Surgery as part of a combined modality approach for inflammatory breast carcinoma. Cancer. 1987 Mar 15;59(6):1063–1067. doi: 10.1002/1097-0142(19870315)59:6<1063::aid-cncr2820590603>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Swain S. M., Lippman M. E. Treatment of patients with inflammatory breast cancer. Important Adv Oncol. 1989:129–150. [PubMed] [Google Scholar]
- Swain S. M., Sorace R. A., Bagley C. S., Danforth D. N., Jr, Bader J., Wesley M. N., Steinberg S. M., Lippman M. E. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res. 1987 Jul 15;47(14):3889–3894. [PubMed] [Google Scholar]
- Thoms W. W., Jr, McNeese M. D., Fletcher G. H., Buzdar A. U., Singletary S. E., Oswald M. J. Multimodal treatment for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 1989 Oct;17(4):739–745. doi: 10.1016/0360-3016(89)90060-6. [DOI] [PubMed] [Google Scholar]
- Wiseman C., Jessup J. M., Smith T. L., Hersh E., Bowen J., Blumenshein G. Inflammatory breast cancer treated with surgery, chemotherapy and allogeneic tumor cell/BCG immunotherapy. Cancer. 1982 Mar 15;49(6):1266–1271. doi: 10.1002/1097-0142(19820315)49:6<1266::aid-cncr2820490631>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]


