Abstract
The pH within individual organelles of the secretory pathway is believed to be an important determinant of their biosynthetic activity. However, little is known about the determinants and regulation of the pH in the secretory organelles, which cannot be readily accessed by [H+]-sensitive probes. We devised a procedure for the dynamic, noninvasive measurement of pH in the lumen of the endoplasmic reticulum in intact mammalian cells. A recombinant form of the B subunit of Shiga toxin, previously modified to include a carboxyl-terminal KDEL sequence and a pH-sensitive fluorophore, was used for a two-stage delivery strategy. Retrograde traffic of endogenous lipids was harnessed to target this protein to the Golgi complex, followed by retrieval to the endoplasmic reticulum (ER) by KDEL receptors. Immunofluorescence and immunoelectron microscopy were used to verify the subcellular localization of the modified B fragment. Fluorescence ratio imaging and two independent calibration procedures were applied to determine the pH of the ER in situ. We found that the pH of the endoplasmic reticulum is near neutral and is unaffected during agonist-induced release of calcium. The ER was found to be highly permeable to H+ (equivalents), so that the prevailing [H+] is susceptible to alterations in the cytosolic pH. Plasmalemmal acid-base transporters were shown to indirectly regulate the endoplasmic reticulum pH.
Protein sorting and targeting during secretion, and retrieval of resident chaperones and receptors are dictated by the prevailing pH in the individual compartments of the secretory pathway. However, the determinants and regulation of the pH within organelles of the secretory pathway, particularly, the endoplasmic reticulum (ER), have remained virtually unexplored, due primarily to our inability to specifically target [H+]-sensitive probes to these compartments. To our knowledge, the only attempts to estimate the pH of the ER were made by quantifying the partition of a permeant weak base by immunoelectron microscopy (1). This approach precludes dynamic analysis of H+ transport and its regulation. The purpose of the work described here was to implement an alternative, spectroscopic method whereby the fluorescence emission of a selectively targeted probe can be used to monitor pH in the ER continuously by ratio imaging.
Some success has been obtained recently targeting pH-sensitive probes to more distal elements of the secretory pathway, by using bacterial toxins. Toxins from Shigella dysenteria and other Shiga-like toxins, such as verotoxin from E. coli, are composed of two types of subunits: a cytotoxic A subunit and a homopentamer of B subunits that serve a targeting role. The B subunits bind to surface glycolipids and are internalized via a retrograde pathway to the Golgi complex (2). The pH of this compartment could be measured optically by covalently attaching [H+]-sensitive fluorophores to the B subunit of verotoxin (3).
Though Shiga and related toxins are thought to exert their biological effects at the ER (2), only a minute fraction of the toxin molecules is normally translocated to the nuclear membrane and peripheral reticulum during the course of hours. This amount was insufficient to measure the pH of the ER. It was apparent that enhanced delivery and/or improved retention of the toxins in the endoplasmic reticulum would be required to obtain adequate signals for measurement of pH. To this end, a recombinant B subunit of Shiga toxin containing the C-terminal ER retrieval signal, KDEL, was used (4). Proteins expressing this tetrapeptide are known to be retrieved from the Golgi complex by receptors that ferry their ligands back to the reticulum (5). The modified recombinant toxin was conjugated covalently to 5-(4,6-dichlorotriazinyl) aminofluorescein. The fluorescence emission of this probe, which is quenched by protons in the physiological range, was suitable to monitor the pH of the ER in intact HeLa cells.
MATERIALS AND METHODS
Reagents and Antibodies.
The acetoxymethyl esters of Fura-2 and BCECF were obtained from Molecular Probes. Trimethylammonium chloride was from Aldrich. Sodium butyrate, lysophosphatidic acid, ouabain, N-methyl-d-glutamine, histamine, and heparin were from Sigma. Concanamycin A was from Kamiya Biomedical (Thousand Oaks, CA). Rabbit polyclonal antibodies to calnexin and α-mannosidase II were generously provided by J. Bergeron (McGill University, Montreal, Canada) and by M. Farquhar (University of California at San Diego, La Jolla), respectively. Antibody to protein disulfide isomerase (PDI) was kindly provided by S. Fuller (European Molecular Biology Laboratory, Heidelberg, Germany). Antibody to the B subunit of Shiga toxin was provided by K. Niebuhr-Ebel (Gesellschaft fuer Biotechnologische Forschung, Braunschweig, Germany).
Preparation and Labeling of Recombinant Toxins.
Recombinant B subunit of verotoxin 1 (VT1B) was produced, affinity-purified and labeled with fluorescein isothiocyanate (FITC) as described (3, 6, 7). A modified recombinant B subunit of verotoxin carrying an exogenous glycosylation site and the tetrapeptide C-terminal sequence, KDEL, was prepared as described recently (4). The modified recombinant B-subunit, referred to hereafter as B-Glyc-KDEL, was purified by using a QFF column (Pharmacia) with linear NaCl elution, followed by Mono Q column (Pharmacia) separation (8). The resulting protein, estimated to be 95% pure by SDS/PAGE, was labeled with 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF) as described earlier (4).
Cells.
HeLa cells obtained from the American Type Culture Collection (Rockville, MD) were grown on glass coverslips (18- or 25-mm diameter, Fisher) to ≈80% confluence in DMEM containing 10% fetal bovine serum, 20 units/ml penicillin, and 20 μg/ml streptomycin under 5% CO2. The cells were then incubated with either 10 μg/ml FITC-labeled VT1B or 1 μg/ml DTAF-labeled B-Glyc-KDEL for 30 min at 4°C. They were subsequently washed in PBS and returned to culture medium for incubation periods between 1.5 and 16 h at 37°C, as specified in the text. Where indicated, the cells were permeabilized by using 0.25 μg/ml of streptolysin O in a buffer containing 10 mM NaCl, 20 mM Hepes, 50 mM KCl, 2 mM K2HPO4, 90 mM K-glutamate, 2 mM CaCl2, 5 mM MgCl2, 4 mM ATP, 3 mM Na pyruvate, 4 mM EGTA, and 100 μg/ml serum albumin, titrated to pH 7.0 or 6.0, as indicated.
Immunofluorescence.
After labeling with toxin, the cells were fixed with 4% paraformaldehyde for 30 min at room temperature and excess fixative was scavenged with 100 mM glycine for 10 min. The cells were next blocked with 5% donkey serum in PBS with 0.1% serum albumin for 1 h at room temperature, washed and incubated with primary antibody (anti-mannosidase II, 1:500; anti-calnexin, 1:400) for 2 h in PBS with albumin. The cells were treated with Cy3-labeled donkey anti-rabbit antibodies (1:1250, Jackson ImmunoResearch). After a final series of washes the coverslip was mounted by using Slow Fade (Molecular Probes). Control experiments were performed in the absence of primary antibody. Samples were viewed with a Leica DM 1RB laser scanning confocal microscope equipped with a 60× objective (Leica). Adobe Systems (Mountain View, CA) photoshop and powerpoint (Microsoft, Irvine, CA) were used to assemble and label the digitized images.
Immunoelectron Microscopy.
Double-labeling immunoelectron microscopy was carried out as described (4). Cryosections of cells that had internalized B-Glyc-KDEL for 6 h were double labeled with two primary antibodies: a polyclonal antibody against the B-fragment of Shiga toxin (1:300) and a mAb to PDI (hybridoma supernatant). Secondary antibodies coupled to different size gold particles were used as detailed in the text and in ref. 4.
Imaging.
Simultaneous bright field and epifluorescence imaging was performed on cells grown on glass coverslips and placed in an Atto Chamber (Molecular Probes) within a thermostatted perfusion holder (Open Perfusion Micro-Incubator; Medical Systems, Greenvale, NY) on the stage of a Zeiss Axiovert I35 inverted microscope (Zeiss). For pH measurements, excitation at 440 and 490 nm was provided by a Xenon arc lamp via computer-controlled shutters and a filter wheel assembly (Sutter Instrument, Novato, CA). The excitation light was attenuated by a neutral density filter and reflected to the cells by a dichroic mirror (510 nm), while the emitted fluorescence (>510 nm) and the transmitted red light (>620 nm) were separated by a second dichroic mirror (580 nm). The red light was directed to a video camera, allowing continuous visualization of the cells, while the fluorescent light was directed onto a 530 nm (15 nm bandpass) filter and captured with a 512 × 512 cooled CCD camera (Princeton Instruments, Princeton, NJ). For cytosolic calcium measurements excitation wavelengths were 340 and 380 nm, and a 400 nm dichroic mirror followed by a 550 nm (40 nm bandpass) filter were used. Control of illumination and image acquisition was achieved by using the Metafluor software (Universal Imaging, Media, PA), operating on a Dell Pentium computer.
Two independent methods were used for in situ calibration of pH. The first used K+-rich buffers and the K+/H+ exchange ionophore, nigericin (5 μg/ml), to equilibrate the endomembrane pH with that of the external medium (125 mM KCl/10 mM Mes/10 mM Hepes/1 mM CaCl2/1 mM MgCl2) (9). Calibration was also performed by using the “null point” method, which utilizes defined mixtures of a permeant weak acid (butyric) and a permeant weak base (trimethylamine) to induce predictable changes in pH (see ref. 10 for details).
RESULTS AND DISCUSSION
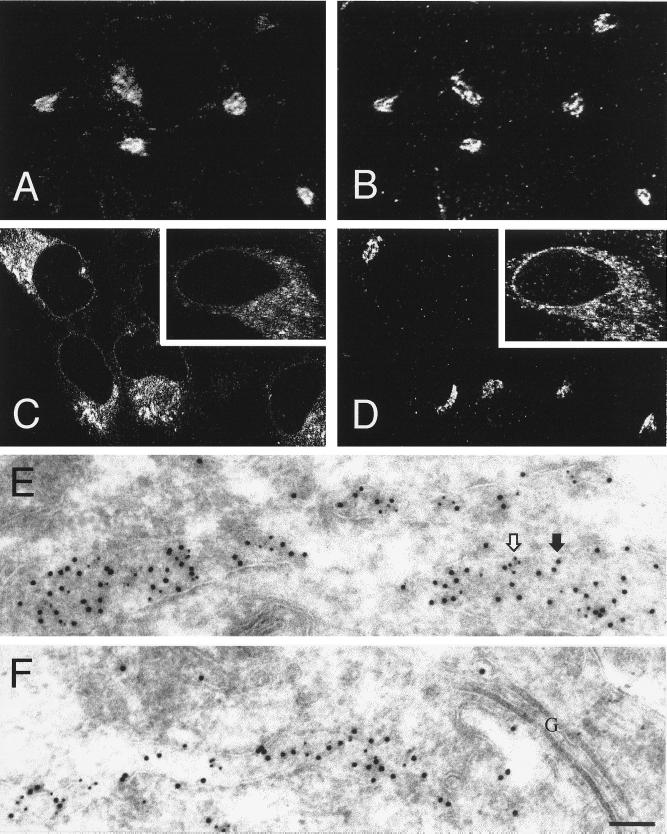
To circumvent the deleterious effects of Shiga toxin, recombinant B subunits devoid of the cytotoxic A subunit were used. To promote their accumulation in the ER, the B subunits were modified to include a C-terminal KDEL sequence (4). In addition, an N-glycosylation site was introduced to verify the location of the toxin by biochemical means. The subcellular distribution of the resulting chimeric protein, termed B-Glyc-KDEL, was initially compared with that of unmodified Shiga-like toxin (VT1B). Following internalization by HeLa cells, VT1B accumulates in a tight juxtanuclear complex that colocalizes with α-mannosidase II (Fig. 1 A and B) and giantin (not shown), markers of Golgi cisternae. Attachment of a KDEL peptide promoted the gradual distribution of Shiga toxin in a diffuse, reticular compartment that was distinctly different from the Golgi stack (Fig. 1 C and D). After ≥6 h, B-Glyc-KDEL colocalized with the ER resident enzyme, protein disulfide isomerase, as shown by dual-label immunoelectron microscopy (Fig. 1 E and F). The toxin also codistributed with calnexin (cf. Fig. 1 C and D Insets) and with the signal sequence receptor (data not shown; see ref. 4), also considered resident proteins of the ER. It is noteworthy that, while at earlier times after internalization B-Glyc-KDEL can be readily detected in Golgi cisternae (data not shown), after ≥6 h the Golgi apparatus is almost devoid of the protein (Fig. 1F). It therefore appears that B-Glyc-KDEL traverses the Golgi complex and is subsequently accumulated effectively by the ER. Accordingly, the acceptor site of B-Glyc-KDEL becomes progressively glycosylated by oligosaccharide transferases that are exclusively located in the ER (4), confirming the subcellular location of the modified toxin.
Figure 1.
Subcellular distribution of native B subunit of Shiga-like toxin (VT1B) and of B-Glyc-KDEL. (A–D) Immunofluorescence confocal microscopy. HeLa cells grown on glass coverslips were incubated with either 10 μg/ml of FITC-labeled VT1B or 1 μg/ml of DTAF-labeled B-Glyc-KDEL for 30 min at 4°C and then incubated for 1.5 h and 16 h, respectively, at 37°C. The cells were then fixed, permeabilized and labeled with organelle-specific marker antibodies. (A) Distribution of VT1B; (B) Immunolocalization of α-mannosidase II in the same cells shown in A; (C) Distribution of B-Glyc-KDEL; (D) Immunolocalization of α-mannosidase II in the same cells shown in C; (C and D Insets) Distribution of B-Glyc-KDEL and immunolocalization of calnexin, respectively. (E and F) Immunoelectron microscopy. Recombinant B-Glyc-KDEL (100 nM) was bound to HeLa cells on ice. The cells were then washed, incubated at 37°C for 6 h and prepared for cryosectioning. The sections were treated with anti-toxin and anti-PDI antibodies followed by gold-labeled secondary antibodies. The location of B-Glyc-KDEL is indicated by 10-nm gold particles (e.g., solid arrow), while PDI is identified by 5-nm gold particles (e.g., open arrow). In F, ER cisternae strongly labeled for B-Glyc-KDEL and PDI were found next to Golgi stacks (denoted by G) that are almost devoid of B-Glyc-KDEL. (Bar = 100 nm.)
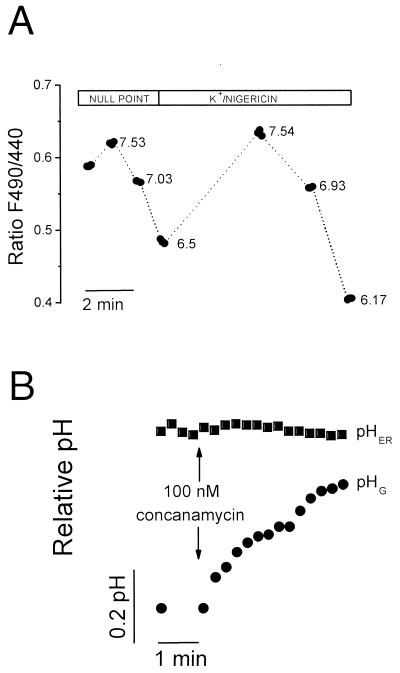
To measure the pH of the ER (pHER), B-Glyc-KDEL was covalently attached to the [H+]-sensitive fluorophore DTAF. In vitro, the conjugate displayed bright fluorescence that was progressively quenched by increasing [H+] with a pKa of ≈6.5, which is suitable for determinations of pH in the physiological range. Targeting of the labeled toxin had no discernible effect on the morphology or function of the ER, assessed as accumulation of calcium and its receptor-mediated release (see below). We therefore proceeded to estimate pHER by using fluorescence ratio imaging. Conventional pH calibration methods are dependent on knowledge of the ionic composition of the compartment under investigation (9). Because the monovalent ion activity within the ER is not defined, we utilized instead a “null-point” procedure that employs a combination of two weak electrolytes of defined pK’s (10). As shown in Fig. 2A, pHER was found to approximate 7.1 in resting HeLa cells. Similar results were obtained by using nigericin and 140 mM K+, suggesting that the K+ activity within the ER is similar to that of the cytosol. In 10 determinations, pHER was stable for at least 20 min and averaged 7.07 ± 0.02 (mean ± SE). By contrast, the pH of the Golgi complex, measured by using unmodified Shiga-like B subunit, was 6.56 ± 0.09 (mean ± SE). The acidity of the Golgi is maintained by a vacuolar-type H+ pump (11), and is therefore dissipated by addition of the specific inhibitor concanamycin (Fig. 2B). Vacuolar pumps seemingly do not play a role in the maintenance of pHER, because addition of concanamycin was without effect. This observation implies that the pumps are not fully assembled or functional in the ER during the course of their biosynthesis.
Figure 2.
Fluorimetric determination of pHER. (A) HeLa cells labeled overnight with DTAF-conjugated B-Glyc-KDEL were excited alternately at 440 nm and 490 nm and the emission was recorded by digital fluorescence microscopy. For null-point calibration, the cells were sequentially perfused with solutions containing varying concentrations of butyrate and trimethylammonium. The pH at which each combination is predicted to equilibrate is indicated. Next, the cells were perfused with K+-rich solutions containing nigericin, at the indicated pH. (B) HeLa cells were labeled either overnight with DTAF-conjugated B-Glyc-KDEL to measure pHER or for 1.5 h with FITC-labeled VT1B to measure the Golgi pH (pHG). Ratio imaging was used for both measurements, as above. Where indicated, 100 nM concanamycin was added to both samples. Representative of five determinations.
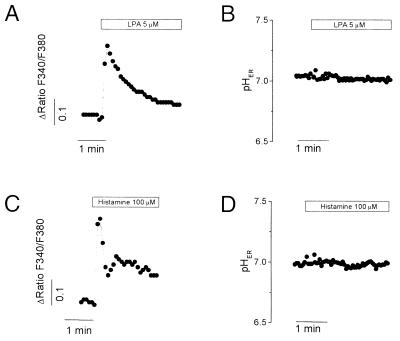
Activation of receptors coupled to phospholipase C results in rapid release of calcium from intracellular stores in HeLa cells (12). In a variety of cells, comparable cytosolic [Ca2+] changes can be accompanied by alterations in cytosolic pH (13). Changes in pHER can also be anticipated due to unmasking of buffering sites upon release of Ca2+ from anionic binding sites and/or due to electrogenic influx driven by Ca2+ diffusion potentials. We therefore undertook measurements of pHER in cells stimulated with either lysophosphatidic acid, histamine or ATP. All the agonists initiated a rapid elevation of cytosolic [Ca2+] that was due to release of intracellular stores, as it persisted in Ca2+-free media (Fig. 3 A and C; ATP results not shown). Parallel determinations indicated that pHER remained stable throughout the period of Ca2+ release (Fig. 3 B and D).
Figure 3.
Measurement of pHER during calcium release. (A and C) Measurements of cytosolic calcium in Fura2-loaded HeLa cells. (B and D) HeLa cells were labeled overnight with DTAF-conjugated B-Glyc-KDEL to measure pHER as in Fig. 2. Where indicated, cells were treated with 5 μM lysophosphatidic acid (LPA) or with 100 μM histamine. Representative of six determinations.
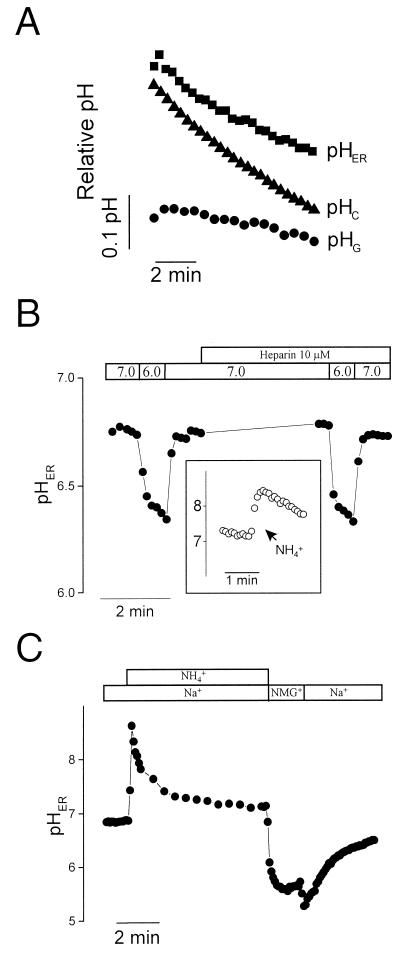
The stability of pHER during the course of Ca2+ release could result from an inordinately high buffering capacity. However, direct measurements by using weak base pulses (14) found the buffering power of the ER in the physiological pH range to be 11.7 mM/pH, similar to that of the cytosol (9.8 mM/pH). Alternatively, the ER membrane may be rather impermeant to H+ (equivalents), precluding electrophoretic translocation. To test the H+ permeability of the ER membrane, we assessed the effects of altering the pH of the surrounding milieu (the cytosol) on pHER. Cytosolic pH was selectively manipulated by operating a plasmalemmal transporter, the Na+/H+ exchanger in its reverse mode, promoting an acute cytosolic acidification. As shown in Fig. 4A, acidification of the cytosolic pH was paralleled by a drop in pHER of comparable time course and slightly smaller magnitude. By comparison, the pH of the Golgi, which is tightly regulated (3), remained largely unaffected during this period (Fig. 4A).
Figure 4.
Effect of cytosolic pH changes on pHER and on Golgi pH. (A) Cytosolic pH (pHC), Golgi pH (pHG) and pHER were measured in separate, parallel experiments by using BCECF, FITC-labeled VT1B and DTAF-conjugated B-Glyc-KDEL, respectively. Cells were Na+-loaded by preincubation in medium with 1 mM ouabain for 45 min. Recording was initiated upon transfer of the cells to a Na+-free medium, pH 5.6, intended to induce cytosolic acidification. (B) HeLa cells labeled with DTAF-conjugated B-Glyc-KDEL were permeabilized with streptolysin O and perfused sequentially with media of pH 6.0 or 7.0, as indicated, while recording pHER. Where noted, 10 μM heparin was added to the solutions. (Inset) effect of NH4+ (40 mM) on pHER in permeabilized cells. (C) Intact cells labeled with DTAF-conjugated B-Glyc-KDEL were bathed in either Na+-rich or Na+-free, isoosmotic N-methyl-d-glucammonium (NMG+) solution, as indicated. Where specified, 40 mM NH4Cl was added to the medium. Representative of six experiments.
These findings suggest that the ER membrane is highly permeable to H+ equivalents. This conclusion was confirmed by selectively permeabilizing the plasmalemma with streptolysin O, to gain direct access to the cytosolic surface of the ER. Step changes in the pH of the permeabilization medium induced rapid and profound changes in pHER. Addition of NH4+/NH3 to such permeabilized cells produced an abrupt alkalosis of pHER (Fig. 4B Inset), resulting from the preferential entry of NH3 and its subsequent protonation in the ER lumen. This observation confirms that the ER membrane remains intact after streptolysin treatment and that B-Glyc-KDEL resides in a compartment distinct from the cytoplasm. Jointly, these results imply that the membrane of the ER is readily traversed by H+ equivalents. Nevertheless, the ER membrane is not generally “leaky,” as it is able to maintain a sizable calcium gradient and has a limited permeability to NH4+, which must enter the ER much more slowly than NH3, as implied by the alkalinization recorded in the inset to Fig. 4B.
Large, inositol 1,4,5-trisphosphate-activated channels mediate the release of Ca2+ in stimulated cells. Some of these are believed to be active in seemingly unstimulated cells, accounting for the release of Ca2+ in cells treated with Ca2+-ATPase blockers like thapsigargin (15, 16). Such channels, however, are not responsible for most of the H+ permeation across the ER membrane, because the pH changes were unaffected by addition of heparin (Fig. 4B), an effective blocker of the inositol trisphosphate activated channels (15–17).
The large H+ permeability of the ER suggests that, unless significant electrical potentials develop across its membrane, pHER would be close to the cytosolic pH at all times. In this event, the effective and multiple pH-regulatory mechanisms that modulate the cytosolic pH would also be indirectly responsible for the maintenance of pHER. This premise was tested by imposing an artificial acid load on the ER (and the cytosol) by application of an NH4+ prepulse. As shown in Fig. 4C, the ER remains acidic when NH4+ is removed in media devoid of extracellular Na+, but rapidly regains near-neutrality when the alkali cation is reintroduced. This behavior is parallel to that of the cytosol (measured independently by using BCECF) and is attributable to activation of the Na+/H+ antiport (18). Thus, a plasmalemmal transporter can indirectly regulate the pHER.
The finding that pHER is similar to the cytosolic pH (i.e., near neutral) has implications on our understanding of the mode of action of bacterial toxins. The inhibition of protein synthesis induced by Shiga and verotoxins is thought to occur in the cytosol, following translocation of their A subunit across the ER membrane (3). This translocation has been postulated to be initiated by an acid-induced change in the conformation of the B subunit (19). Our finding that pHER is neutral suggests that either such a conformational change is not necessary, or that translocation of the A subunit to the cytosol occurs from a compartment other than the ER. Support for the latter assumption comes from preliminary experiments that show that holotoxin reconstituted combining B-Glyc-KDEL with wild-type A subunit is no more cytotoxic than a comparable holotoxin reconstituted by using the structurally analogous B-Glyc-KDELGL (C.L. and L.J., unpublished observations). The latter form of the B subunit is not retrieved by the KDEL receptor and therefore, like wild-type B subunit in HeLa cells, accumulates in the Golgi complex (4).
In summary, we have described a sensitive method for the continuous measurement of pHER. In HeLa cells, pHER was found to be similar to the cytosolic pH and to be indirectly regulated by transporters on the plasma membrane. The large H+ permeability of the ER membrane effectively connects this compartment with the buffer reservoir of the cytoplasm and with the homeostatic mechanisms that control cytosolic pH. This may account for the stability of pHER during the course of Ca2+ release and reuptake. Finally, it is likely that the strategy used here to monitor pH in the ER can be extended to measure other ionic species, by coupling appropriate spectroscopic probes to B-Glyc-KDEL.
Acknowledgments
We thank D. Tenza for her expertise in cryosectioning and immunogold labeling. This work was supported by grants from the Canadian Cystic Fibrosis Foundation and the Medical Research Council of Canada. J.H.K. was supported by the Canadian Cystic Fibrosis Foundation, Medical Research Council, and Janssen-Ortho Pharmaceuticals. S.G. is an International Scholar of the Howard Hughes Medical Institute.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BCECF, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein; B-Glyc-KDEL, B subunit of Shiga toxin modified to include a glycosylation site and a C-terminal KDEL sequence; DTAF, 5-(4,6-dichlorotriazinyl)aminofluorescein; ER, endoplasmic reticulum; FITC, fluorescein isothiocyanate; PDI, protein disulfide isomerase; pHER, endoplasmic reticulum pH; pHG, Golgi pH; VT1B, B subunit of Shiga-like toxin (verotoxin 1).
References
- 1.Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli J-D, Anderson R G. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandvig K, van Deurs B. Physiol Rev. 1996;76:949–966. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- 3.Kim J H, Lingwood C A, Williams D B, Furuya W, Manolson M F, Grinstein S. J Cell Biol. 1996;134:1387–1399. doi: 10.1083/jcb.134.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannes L, Tenza D, Antony C, Goud B. J Biol Chem. 1997;272:19554–19561. doi: 10.1074/jbc.272.31.19554. [DOI] [PubMed] [Google Scholar]
- 5.Dean N, Pelham H R. J Cell Biol. 1990;111:369–377. doi: 10.1083/jcb.111.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramotar K, Boyd B, Tyrrell G, Gariepy J, Lingwood C, Brunton J. Biochem J. 1990;272:805–811. doi: 10.1042/bj2720805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulanger J, Huesca M, Arab S, Lingwood C A. Anal Biochem. 1994;217:1–6. doi: 10.1006/abio.1994.1075. [DOI] [PubMed] [Google Scholar]
- 8.Su G F, Brahmbhatt H N, Wehland J, Rohde M, Timmis K N. Infect Immun. 1992;60:3345–3359. doi: 10.1128/iai.60.8.3345-3359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas J A, Buchsbaum R N, Zimniak A, Racker E. Biochemistry. 1982;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 10.Eisner D A, Kenning N A, O’Neill S C, Pocock G, Valdeolmillos M. Eur J Physiol. 1989;413:553–558. doi: 10.1007/BF00594188. [DOI] [PubMed] [Google Scholar]
- 11.Glickman J, Croen K, Kelly S, Al-Awqati Q. J Cell Biol. 1983;97:1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilly B C, Tertoolen L G, Lambrechts A C, Remorie R, de Laat S W, Moolenaar W H. Biochem J. 1990;266:235–243. doi: 10.1042/bj2660235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busa W B, Nuccitelli R. Am J Physiol. 1984;246:R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- 14.Szatkowski M S, Thomas R C. Pflügers Arch. 1986;407:59–63. doi: 10.1007/BF00580721. [DOI] [PubMed] [Google Scholar]
- 15.Toescu E C, Petersen O H. Pflügers Arch. 1994;427:325–331. doi: 10.1007/BF00374541. [DOI] [PubMed] [Google Scholar]
- 16.Favre C J, Lew D P, Krause K H. Biochem J. 1994;302:155–162. doi: 10.1042/bj3020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh T K, Eis P S, Mullaney J M, Ebert C L, Gill D L. J Biol Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- 18.Orlowski J, Grinstein S. J Biol Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 19.Saleh M T, Gariepy J. Biochemistry. 1993;32:918–922. doi: 10.1021/bi00054a024. [DOI] [PubMed] [Google Scholar]