Abstract
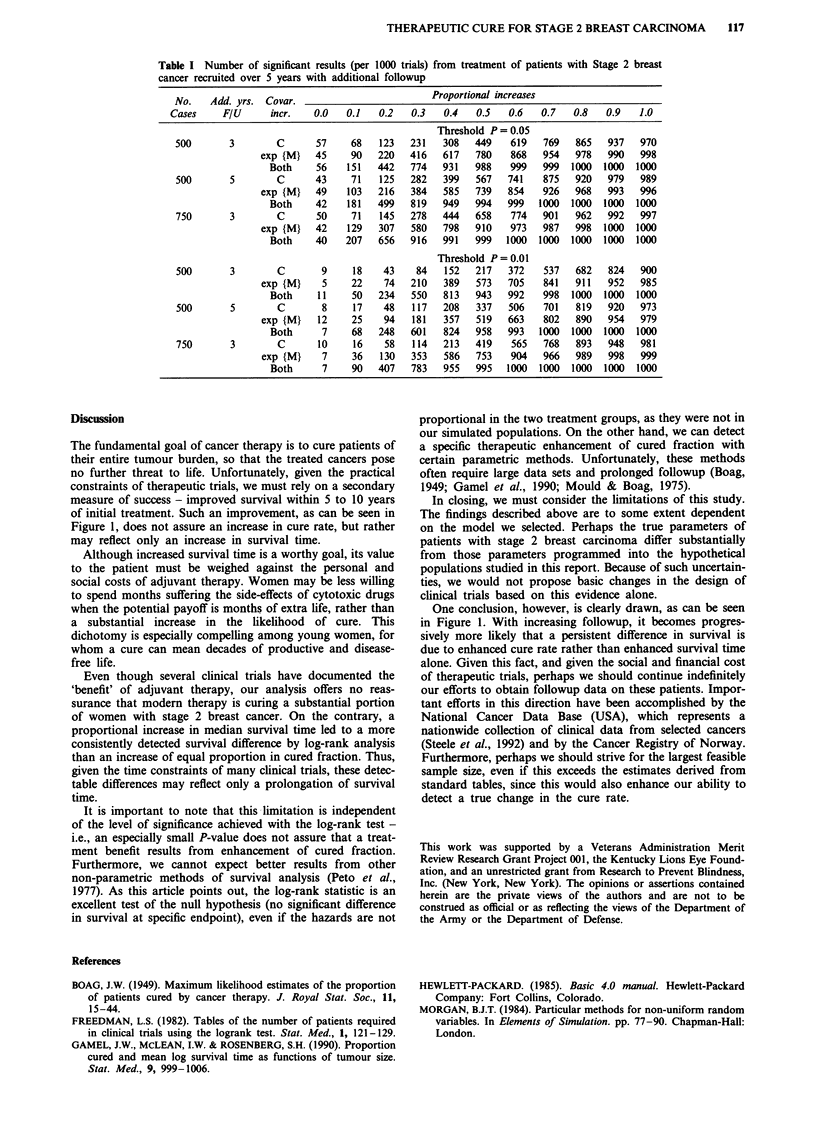
The log-rank test is commonly used to assess therapeutic effect in prospective, randomised clinical trials. This test is sensitive to differences in survival between treatment groups at a specific endpoint, but cannot determine whether such a difference is due to an enhanced cure rate or an enhanced survival time among uncured patients. To investigate the clinical impact of such limitations, an algorithm was constructed to simulate clinical, randomised, adjuvant therapy trials in patients with a cured fraction of 0.27 and a median survival time for uncured patients of 3.4 years. Hypothetical therapies were introduced to increase rate of cure, increase median survival time, or achieve a combination of these effects. For 500 simulated patients recruited over a 5 year period and then followed for three additional years, a 50% enhancement of median survival time (to 5.1 years) led to a survival increase detectable at the P = 0.05 level in 780 of 1000 trials, whereas a 50% enhancement of cured fraction (to 40.5%) led to a detectable increase at the same level in only 449 of 1000 trials. These findings suggest that, in clinical trials of adjuvant therapy for stage 2 breast cancer, the log rank test may be more sensitive to increases in tumour-related survival time than to increases in cured fraction.
Full text
PDF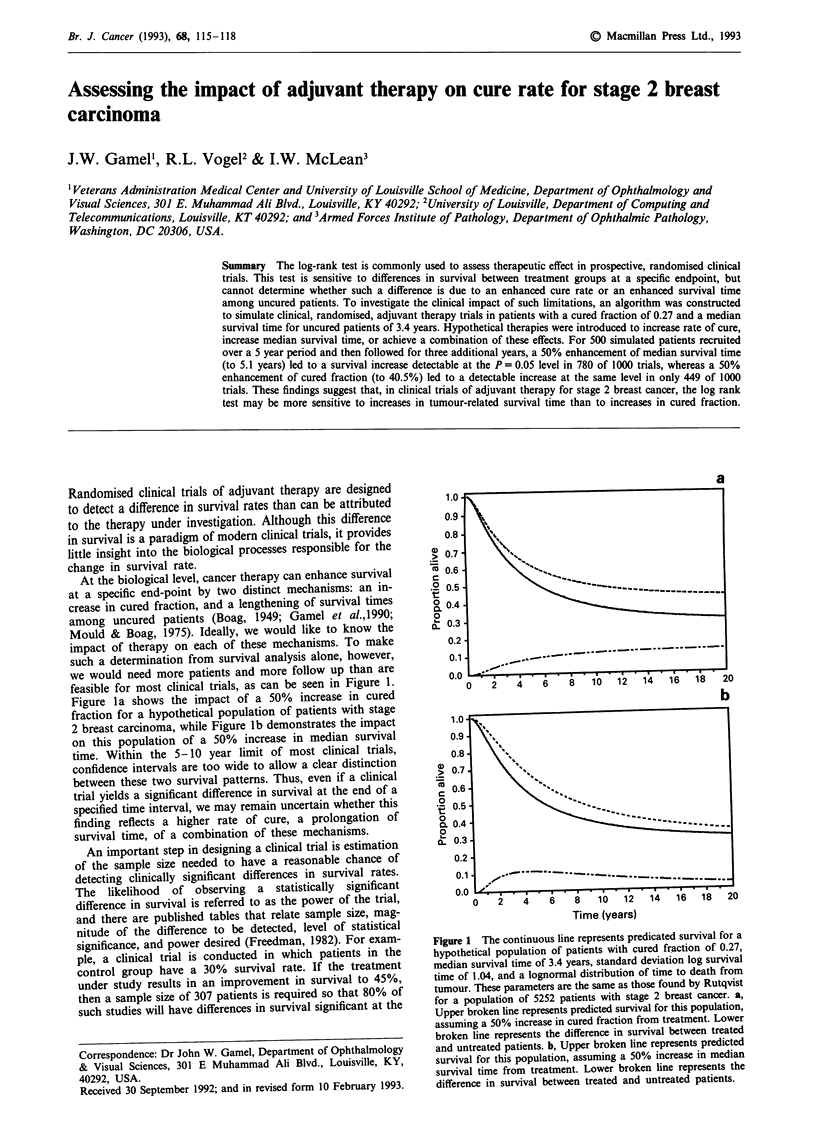


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freedman L. S. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982 Apr-Jun;1(2):121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- Gamel J. W., McLean I. W., Rosenberg S. H. Proportion cured and mean log survival time as functions of tumour size. Stat Med. 1990 Aug;9(8):999–1006. doi: 10.1002/sim.4780090814. [DOI] [PubMed] [Google Scholar]
- Mould R. F., Boag J. W. A test of several parametic statistical models for estimating success rate in the treatment of carcinoma cervix uteri. Br J Cancer. 1975 Nov;32(5):529–550. doi: 10.1038/bjc.1975.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutqvist L. E., Wallgren A., Nilsson B. Is breast cancer a curable disease? A study of 14,731 women with breast cancer from the Cancer Registry of Norway. Cancer. 1984 Apr 15;53(8):1793–1800. doi: 10.1002/1097-0142(19840415)53:8<1793::aid-cncr2820530832>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]



