Abstract
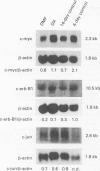
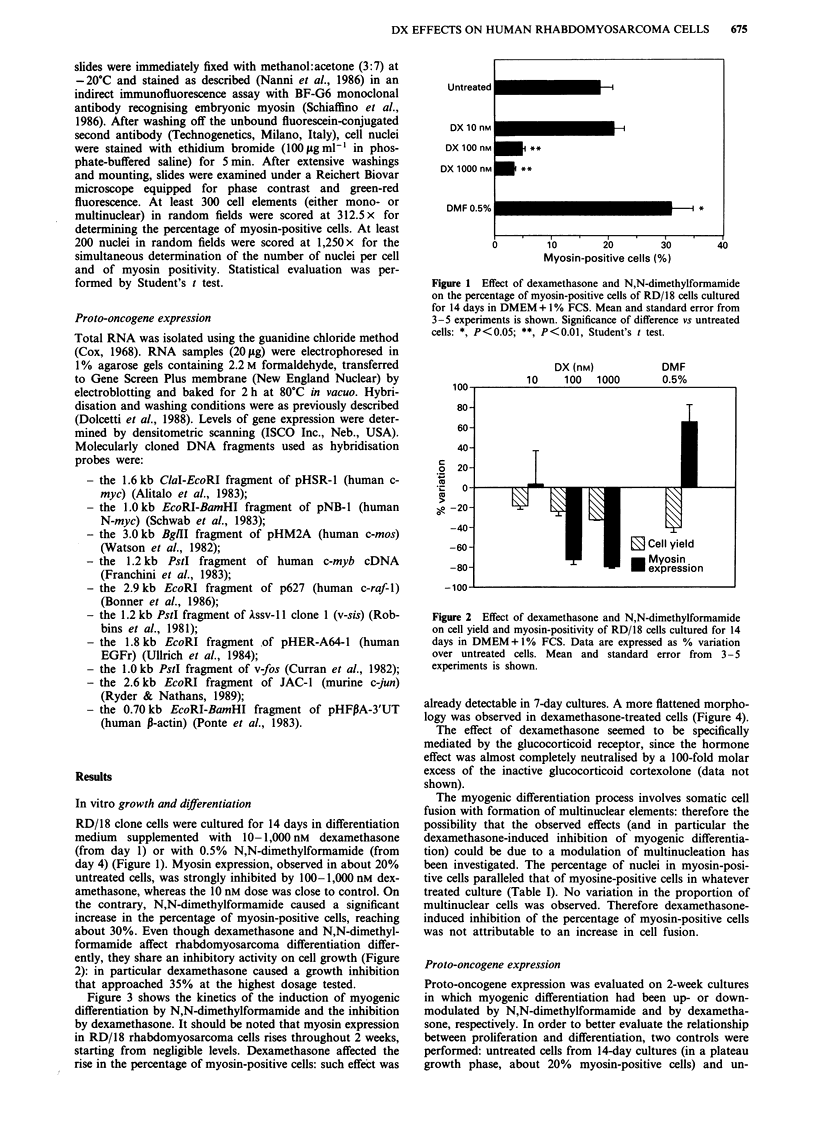
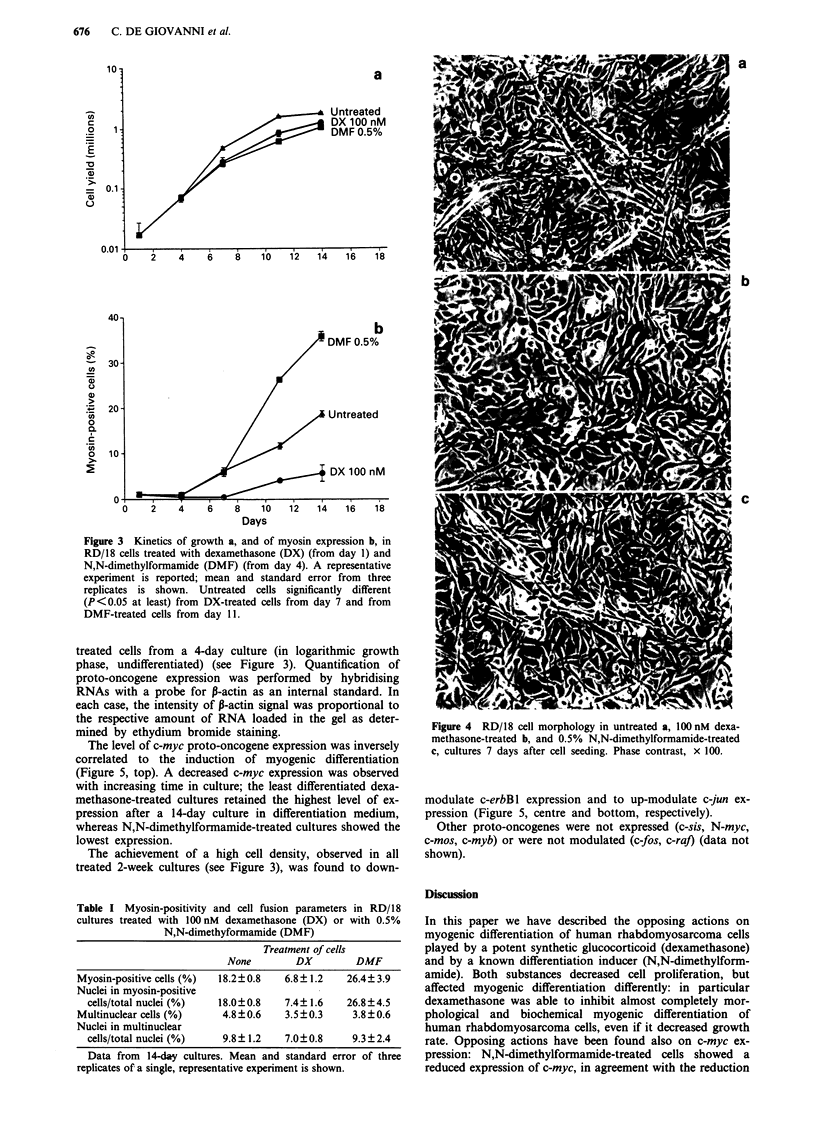
The effects of dexamethasone, a synthetic glucocorticoid, and of N,N-dimethylformamide on in vitro growth and differentiation and on proto-oncogene expression of human rhabdomyosarcoma cells were studied. RD/18 clone cells (derived from the embryonal rhabdomyosarcoma cell line RD) treated with 100 nM dexamethasone showed an almost complete block of differentiation: about 5% myosin-positive cells were observed after 2 weeks of culture in dexamethasone-supplemented differentiation medium, compared to 20% of untreated cultures. Dexamethasone also induced a 20-30% growth inhibition and a more flattened morphology. The treatment with N,N-dimethylformamide induced a significantly increased proportion of myosin-positive cells (reaching about 30%) and a 40% growth inhibition. Induction of differentiation inversely correlated with the levels of c-myc proto-oncogene expression: after a 2 week culture dexamethasone-treated cells showed the highest c-myc expression and N,N-dimethylformamide-treated cells the lowest. Culture conditions per se down-modulated c-erbB1 and up-regulated c-jun expression, with no relationship to the differentiation pattern. Other proto-oncogenes were not expressed (c-sis, N-myc, c-mos, c-myb) or were not modulated (c-fos, c-raf). Therefore dexamethasone and N,N-dimethylformamide, both causing a decreased growth rate, showed opposing actions on myogenic differentiation and on c-myc proto-oncogene expression of human rhabdomyosarcoma cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguanno S., Bouchè M., Adamo S., Molinaro M. 12-O-tetradecanoylphorbol-13-acetate-induced differentiation of a human rhabdomyosarcoma cell line. Cancer Res. 1990 Jun 1;50(11):3377–3382. [PubMed] [Google Scholar]
- Alemá S., Tató F. Interaction of retroviral oncogenes with the differentiation program of myogenic cells. Adv Cancer Res. 1987;49:1–28. doi: 10.1016/s0065-230x(08)60792-7. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Lazo J. S. Suppression of dexamethasone-induced metallothionein expression and cis-diamminedichloroplatinum(II) resistance by v-mos. Cancer Res. 1991 Feb 1;51(3):893–896. [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Oppermann H., Seeburg P., Kerby S. B., Gunnell M. A., Young A. C., Rapp U. R. The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gene. Nucleic Acids Res. 1986 Jan 24;14(2):1009–1015. doi: 10.1093/nar/14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C., Lanson N. A., Jr Proto-oncogene expression in proliferating and differentiating cardiac and skeletal muscle. Biochem J. 1987 Nov 1;247(3):701–706. doi: 10.1042/bj2470701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dexter D. L. N,N-Dimethylformamide-induced morphological differentiation and reduction of tumorigenicity in cultured mouse rhabdomyosarcoma cells. Cancer Res. 1977 Sep;37(9):3136–3140. [PubMed] [Google Scholar]
- Dolcetti R., De Re V., Viel A., Pistello M., Tavian M., Boiocchi M. Nuclear oncogene amplification or rearrangement is not involved in human colorectal malignancies. Eur J Cancer Clin Oncol. 1988 Aug;24(8):1321–1328. doi: 10.1016/0277-5379(88)90223-4. [DOI] [PubMed] [Google Scholar]
- Dym H., Yaffe D. Expression of creatine kinase isoenzymes in myogenic cell lines. Dev Biol. 1979 Feb;68(2):592–599. doi: 10.1016/0012-1606(79)90229-x. [DOI] [PubMed] [Google Scholar]
- Florini J. R. Hormonal control of muscle growth. Muscle Nerve. 1987 Sep;10(7):577–598. doi: 10.1002/mus.880100702. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Magri K. A. Effects of growth factors on myogenic differentiation. Am J Physiol. 1989 Apr;256(4 Pt 1):C701–C711. doi: 10.1152/ajpcell.1989.256.4.C701. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Baluda M. A., Lengel C., Tronick S. R. Structural organization and expression of human DNA sequences related to the transforming gene of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7385–7389. doi: 10.1073/pnas.80.24.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbert H. E., Gerharz C. D., Biesalski H. K., Engers R., Luley C. Terminal differentiation and growth inhibition of a rat rhabdomyosarcoma cell line (BA-HAN-1C) in vitro after exposure to retinoic acid. Cancer Res. 1988 Sep 15;48(18):5264–5269. [PubMed] [Google Scholar]
- Gabbert H. E., Gerharz C. D., Ramp U., Hoffmann J., Oster O., Oesch F., Doehmer J. Enhanced expression of the proto-oncogenes fos and raf in the rhabdomyosarcoma cell line BA-HAN-1C after differentiation induction with retinoic acid and N-methylformamide. Int J Cancer. 1990 Apr 15;45(4):724–730. doi: 10.1002/ijc.2910450426. [DOI] [PubMed] [Google Scholar]
- Garvin A. J., Stanley W. S., Bennett D. D., Sullivan J. L., Sens D. A. The in vitro growth, heterotransplantation, and differentiation of a human rhabdomyosarcoma cell line. Am J Pathol. 1986 Oct;125(1):208–217. [PMC free article] [PubMed] [Google Scholar]
- Gerharz C. D., Gabbert H. E., Biesalski H. K., Engers R., Luley C. Fetal calf serum and retinoic acid affect proliferation and terminal differentiation of a rat rhabdomyosarcoma cell line (BA-HAN-1C). Br J Cancer. 1989 Jan;59(1):61–67. doi: 10.1038/bjc.1989.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerharz C. D., Gabbert H. E., Engers R., Ramp U., Mayer H., Luley C. Heterogeneous response to differentiation induction with different polar compounds in a clonal rat rhabdomyosarcoma cell line (BA-HAN-1C). Br J Cancer. 1989 Oct;60(4):578–584. doi: 10.1038/bjc.1989.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero V., Jr, Florini J. R. Dexamethasone effects on myoblast proliferation and differentiation. Endocrinology. 1980 Apr;106(4):1198–1202. doi: 10.1210/endo-106-4-1198. [DOI] [PubMed] [Google Scholar]
- Hamilton B. J., DeFranco D. Glucocorticoid and cAMP induction mechanisms are differentially affected by the p85gag-mos oncoprotein. Proc Natl Acad Sci U S A. 1989 Jan;86(2):597–601. doi: 10.1073/pnas.86.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan I., Blakely B. T., Pavlath G. K., Travis M., Blau H. M. Steroids induce acetylcholine receptors on cultured human muscle: implications for myasthenia gravis. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8100–8104. doi: 10.1073/pnas.87.20.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch M. P., Leibovitch S. A., Hillion J., Guillier M., Schmitz A., Harel J. Possible role of c-fos, c-N-ras and c-mos proto-oncogenes in muscular development. Exp Cell Res. 1987 May;170(1):80–92. doi: 10.1016/0014-4827(87)90118-2. [DOI] [PubMed] [Google Scholar]
- Lim R. W., Hauschka S. D. A rapid decrease in epidermal growth factor-binding capacity accompanies the terminal differentiation of mouse myoblasts in vitro. J Cell Biol. 1984 Feb;98(2):739–747. doi: 10.1083/jcb.98.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollini P. L., De Giovanni C., Del Re B., Landuzzi L., Nicoletti G., Prodi G., Scotlandi K., Nanni P. Myogenic differentiation of human rhabdomyosarcoma cells induced in vitro by antineoplastic drugs. Cancer Res. 1989 Jul 1;49(13):3631–3636. [PubMed] [Google Scholar]
- Lollini P. L., De Giovanni C., Landuzzi L., Nicoletti G., Scotlandi K., Nanni P. Reduced metastatic ability of in vitro differentiated human rhabdomyosarcoma cells. Invasion Metastasis. 1991;11(2):116–124. [PubMed] [Google Scholar]
- Matin A., Cheng K. L., Suen T. C., Hung M. C. Effect of glucocorticoids on oncogene transformed NIH3T3 cells. Oncogene. 1990 Jan;5(1):111–116. [PubMed] [Google Scholar]
- Max S. R., Thomas J. W., Banner C., Vitkovic L., Konagaya M., Konagaya Y. Glucocorticoid receptor-mediated induction of glutamine synthetase in skeletal muscle cells in vitro. Endocrinology. 1987 Mar;120(3):1179–1183. doi: 10.1210/endo-120-3-1179. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Ito H., Blau H. M., Torti F. M. Tumor necrosis factor inhibits human myogenesis in vitro. Mol Cell Biol. 1988 Jun;8(6):2295–2301. doi: 10.1128/mcb.8.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Wold B. J. c-myc inhibition of MyoD and myogenin-initiated myogenic differentiation. Mol Cell Biol. 1991 May;11(5):2842–2851. doi: 10.1128/mcb.11.5.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni P., Schiaffino S., De Giovanni C., Nicoletti G., Prodi G., Del Re B., Eusebi V., Ceccarelli C., Saggin L., Lollini P. L. RMZ: a new cell line from a human alveolar rhabdomyosarcoma. In vitro expression of embryonic myosin. Br J Cancer. 1986 Dec;54(6):1009–1014. doi: 10.1038/bjc.1986.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti G., De Giovanni C., Landuzzi L., Simone G., Rocchi P., Nanni P., Lollini P. L. Induction of myogenic differentiation in human rhabdomyosarcoma cells by ionising radiation, N,N-dimethylformamide and their combination. Br J Cancer. 1992 Apr;65(4):519–522. doi: 10.1038/bjc.1992.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Brennan T. J., Chakraborty T., Cheng T. C., Cserjesi P., Edmondson D., James G., Li L. Molecular control of myogenesis: antagonism between growth and differentiation. 1991 May 29-Jun 12Mol Cell Biochem. 104(1-2):7–13. doi: 10.1007/BF00229797. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon M., Megyesi J., Green R. S., Zhang H., Rollins B. J., Safirstein R., Taubman M. B. In vivo and in vitro inhibition of JE gene expression by glucocorticoids. J Biol Chem. 1991 Nov 25;266(33):22375–22379. [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Aaronson S. A. Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones. Proc Natl Acad Sci U S A. 1981 May;78(5):2918–2922. doi: 10.1073/pnas.78.5.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Carli M. Embryonic myosin heavy chain as a differentiation marker of developing human skeletal muscle and rhabdomyosarcoma. A monoclonal antibody study. Exp Cell Res. 1986 Mar;163(1):211–220. doi: 10.1016/0014-4827(86)90574-4. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Sklar R. M., Brown R. H., Jr Methylprednisolone increases dystrophin levels by inhibiting myotube death during myogenesis of normal human muscle in vitro. J Neurol Sci. 1991 Jan;101(1):73–81. doi: 10.1016/0022-510x(91)90019-4. [DOI] [PubMed] [Google Scholar]
- Southorn B. G., Palmer R. M. Inhibitors of phospholipase A2 block the stimulation of protein synthesis by insulin in L6 myoblasts. Biochem J. 1990 Sep 15;270(3):737–739. doi: 10.1042/bj2700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson P. A., Stuart C. A., Huls M. H., Sams C. F., Cintron N. M. Dexamethasone effects on creatine kinase activity and insulin-like growth factor receptors in cultured muscle cells. J Cell Physiol. 1989 Jul;140(1):8–17. doi: 10.1002/jcp.1041400103. [DOI] [PubMed] [Google Scholar]