Abstract
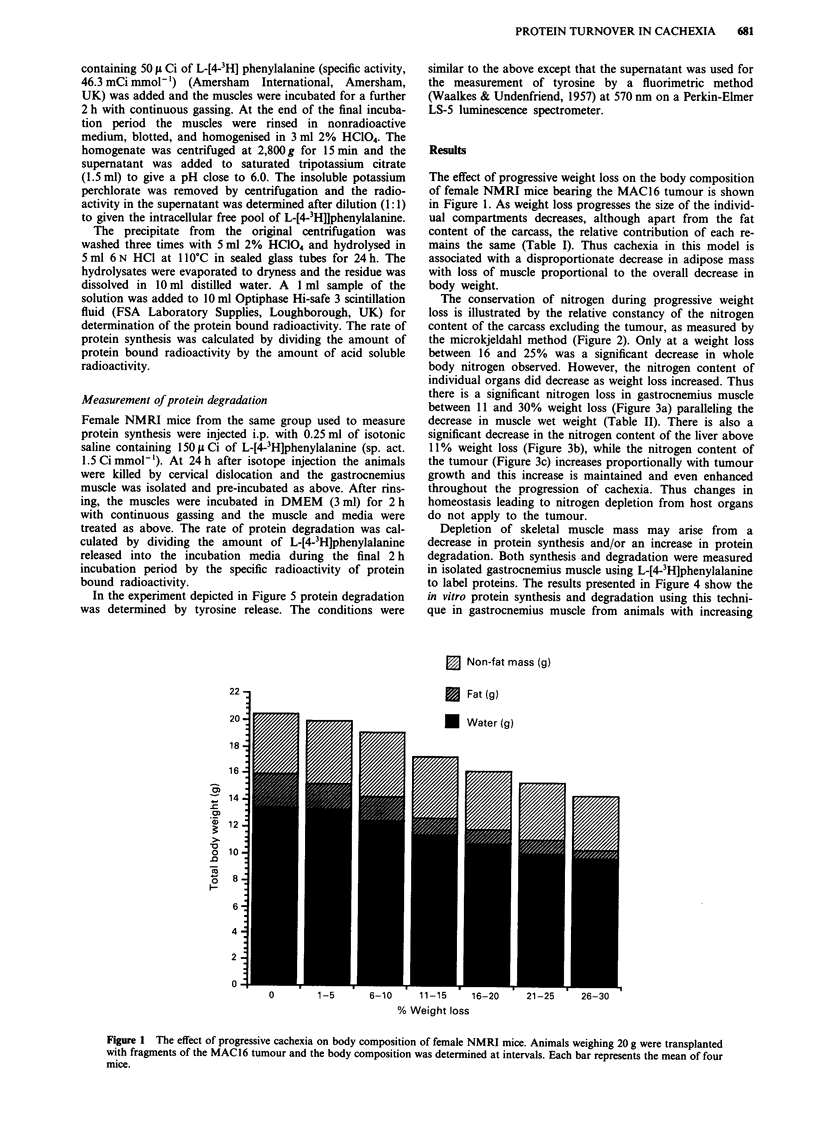
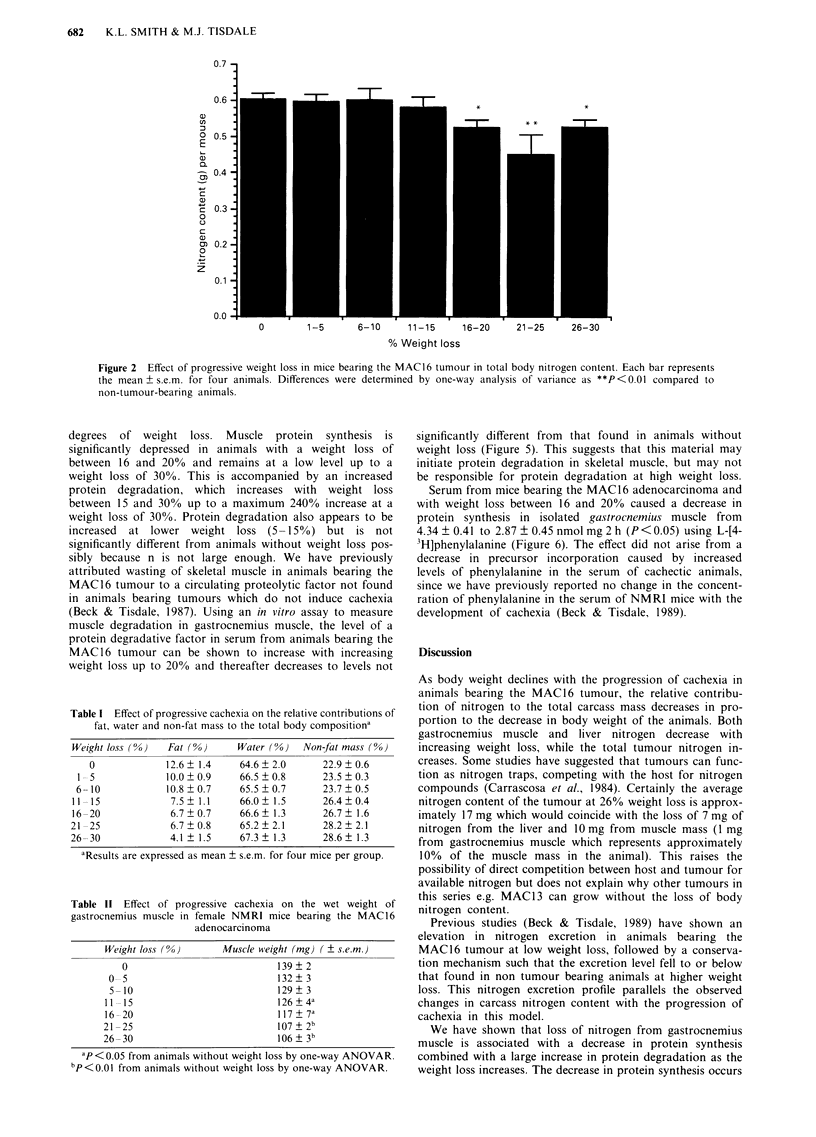
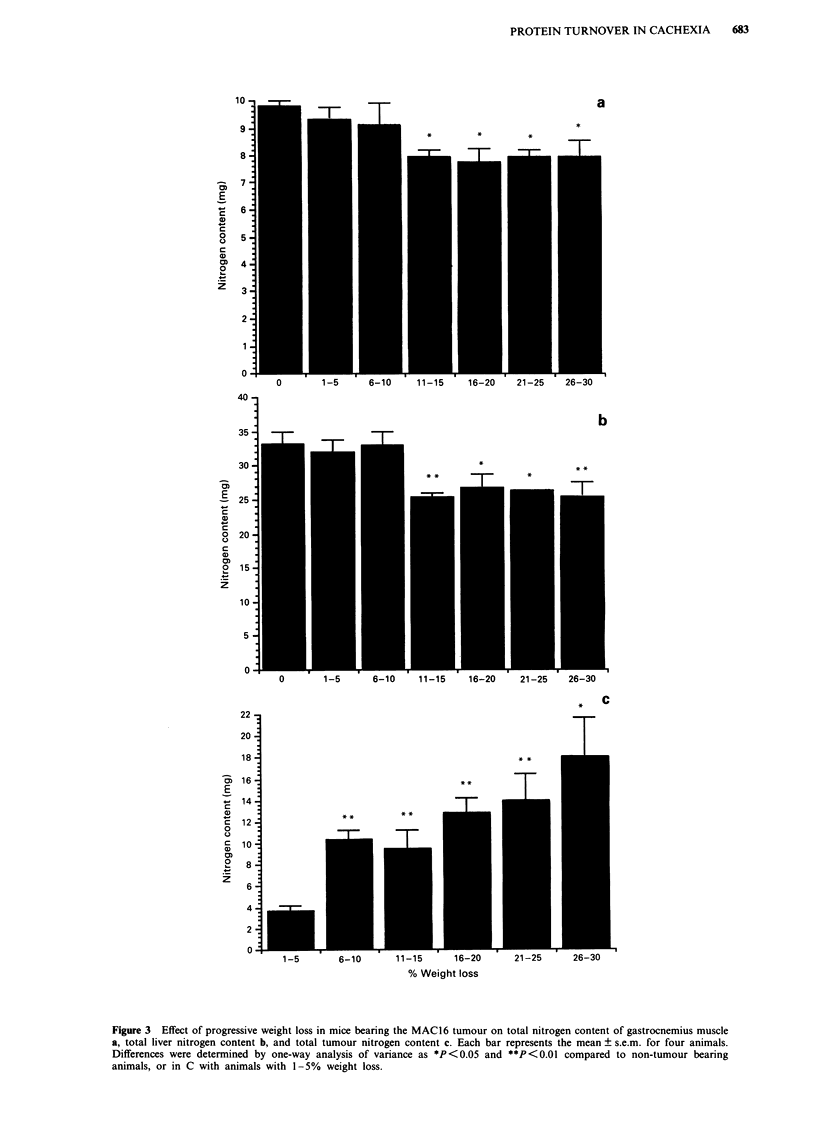
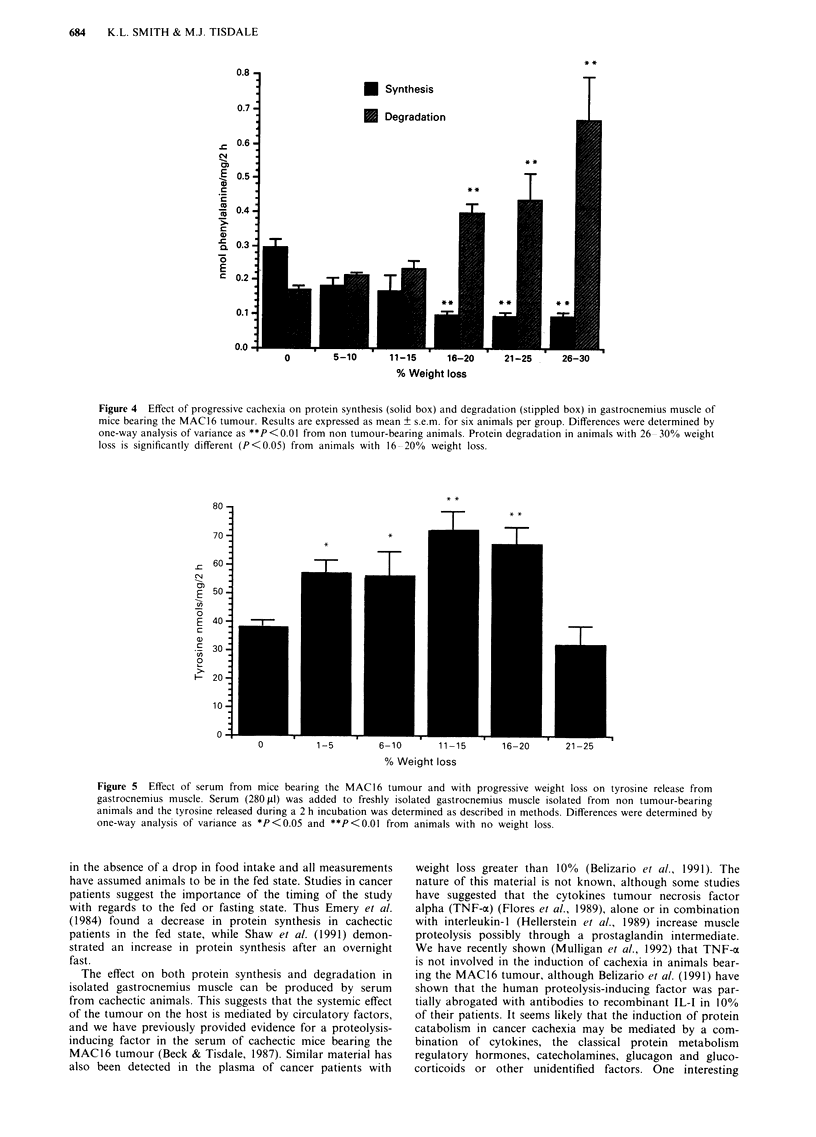
The effects of progressive cachexia on protein metabolism in skeletal muscle has been investigated in mice bearing the MAC16 adenocarcinoma which produces cachexia with tumour burdens of < 1% of the host weight. Weight loss was accompanied by loss of whole body nitrogen in proportion to the overall loss of body mass. Using L-[4-3H]phenylalanine to label proteins in gastrocnemius muscle, a significant depression (60%) in protein synthesis occurred in animals with a weight loss between 15 and 30% accompanied by an increase in protein degradation, which increased with increasing weight loss between 15 and 30%. Muscle degradation in vitro could be achieved by serum from cachectic animals, which appeared to contain a proteolysis-inducing factor. These results suggest that the increased degradation of skeletal muscle seen in this model of cachexia may be due to a circulating proteolysis-inducing factor.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V. E., Goldberg A. L. Maintenance of normal length improves protein balance and energy status in isolated rat skeletal muscles. Am J Physiol. 1986 Oct;251(4 Pt 1):C588–C596. doi: 10.1152/ajpcell.1986.251.4.C588. [DOI] [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Nitrogen excretion in cancer cachexia and its modification by a high fat diet in mice. Cancer Res. 1989 Jul 15;49(14):3800–3804. [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res. 1987 Nov 15;47(22):5919–5923. [PubMed] [Google Scholar]
- Belizario J. E., Katz M., Chenker E., Raw I. Bioactivity of skeletal muscle proteolysis-inducing factors in the plasma proteins from cancer patients with weight loss. Br J Cancer. 1991 May;63(5):705–710. doi: 10.1038/bjc.1991.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby M. C., Double J. A., Ali S. A., Fearon K. C., Brennan R. A., Tisdale M. J. Characterization of a transplantable adenocarcinoma of the mouse colon producing cachexia in recipient animals. J Natl Cancer Inst. 1987 Mar;78(3):539–546. [PubMed] [Google Scholar]
- Carrascosa J. M., Martínez P., Núez de Castro I. Nitrogen movement between host and tumor in mice inoculated with Ehrlich ascitic tumor cells. Cancer Res. 1984 Sep;44(9):3831–3835. [PubMed] [Google Scholar]
- Clark C. M., Goodlad G. A. Depletion of proteins of phasic and tonic muscles in tumour-bearing rats. Eur J Cancer. 1971 Feb;7(1):3–9. doi: 10.1016/0014-2964(71)90088-0. [DOI] [PubMed] [Google Scholar]
- Emery P. W., Edwards R. H., Rennie M. J., Souhami R. L., Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 1984 Sep 8;289(6445):584–586. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E. A., Bistrian B. R., Pomposelli J. J., Dinarello C. A., Blackburn G. L., Istfan N. W. Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest. 1989 May;83(5):1614–1622. doi: 10.1172/JCI114059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein M. K., Meydani S. N., Meydani M., Wu K., Dinarello C. A. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Invest. 1989 Jul;84(1):228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm K., Bylund A. C., Holm J., Scherstén T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976 Jun;12(6):465–473. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- Norton J. A., Shamberger R., Stein T. P., Milne G. W., Brennan M. F. The influence of tumor-bearing on protein metabolism in the rat. J Surg Res. 1981 May;30(5):456–462. doi: 10.1016/0022-4804(81)90090-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Ogden J., Ramjee G., Rund J. Contribution of elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. Cancer Res. 1990 Feb 15;50(4):1226–1230. [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rotman E. I., Brostrom M. A., Brostrom C. O. Inhibition of protein synthesis in intact mammalian cells by arachidonic acid. Biochem J. 1992 Mar 1;282(Pt 2):487–494. doi: 10.1042/bj2820487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Humberstone D. A., Douglas R. G., Koea J. Leucine kinetics in patients with benign disease, non-weight-losing cancer, and cancer cachexia: studies at the whole-body and tissue level and the response to nutritional support. Surgery. 1991 Jan;109(1):37–50. [PubMed] [Google Scholar]
- Strelkov A. B., Fields A. L., Baracos V. E. Effects of systemic inhibition of prostaglandin production on protein metabolism in tumor-bearing rats. Am J Physiol. 1989 Aug;257(2 Pt 1):C261–C269. doi: 10.1152/ajpcell.1989.257.2.C261. [DOI] [PubMed] [Google Scholar]
- Tessitore L., Bonelli G., Baccino F. M. Early development of protein metabolic perturbations in the liver and skeletal muscle of tumour-bearing rats. A model system for cancer cachexia. Biochem J. 1987 Jan 1;241(1):153–159. doi: 10.1042/bj2410153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Wu G. Y., Thompson J. R. The effect of ketone bodies on alanine and glutamine metabolism in isolated skeletal muscle from the fasted chick. Biochem J. 1988 Oct 1;255(1):139–144. doi: 10.1042/bj2550139. [DOI] [PMC free article] [PubMed] [Google Scholar]



