Abstract
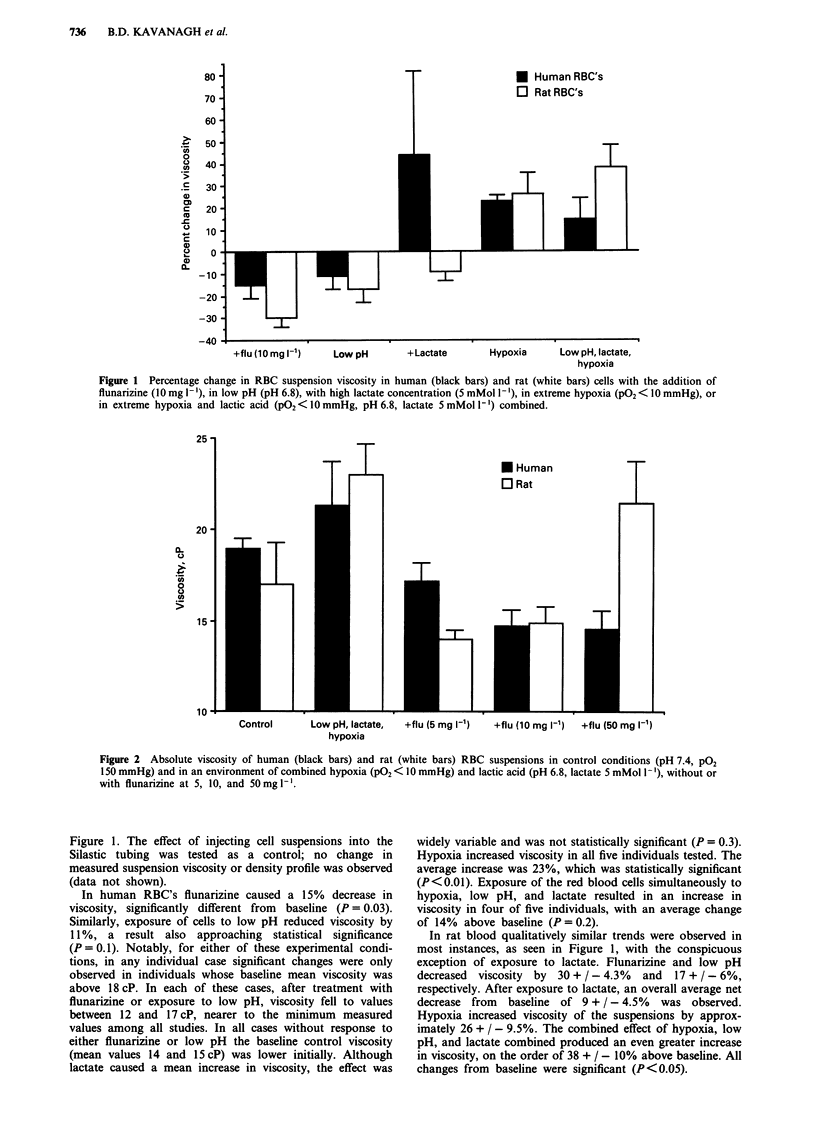
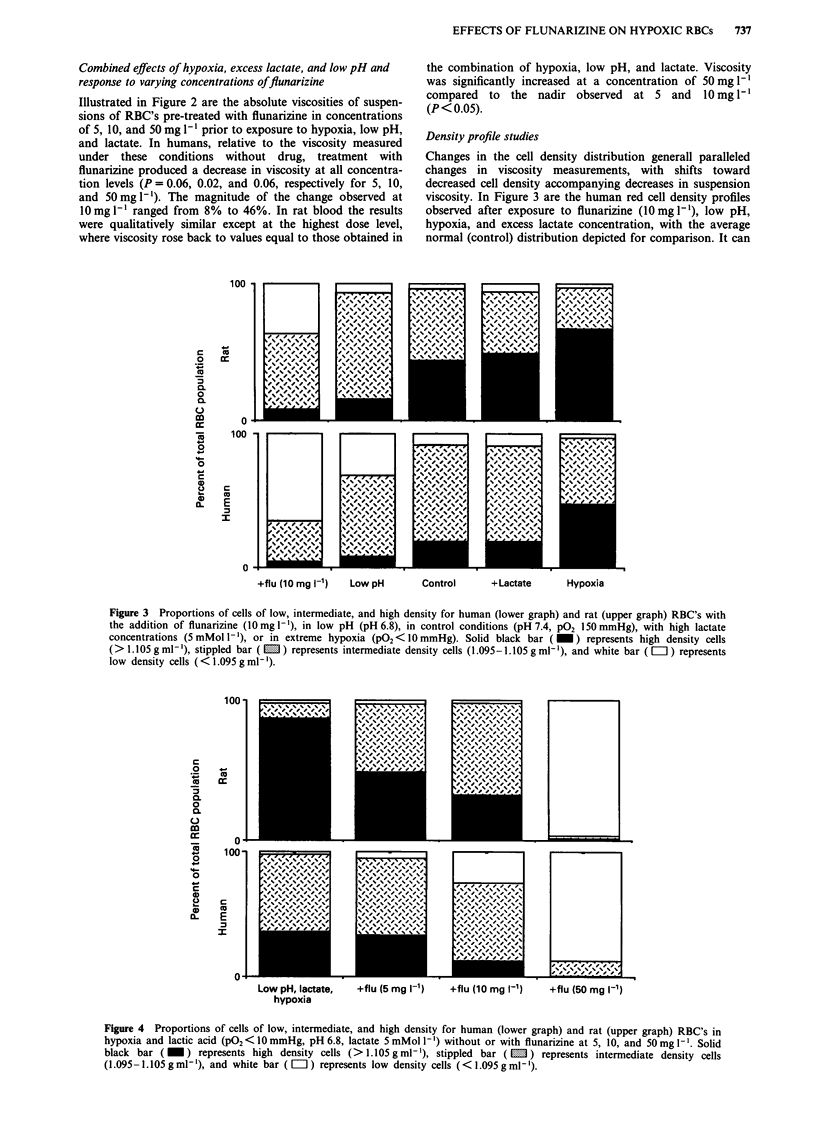
Flunarizine is a class IV calcium channel blocker which increases oxygen delivery to hypoxic regions in solid tumours, exerting a radiosensitising effect in vivo in animal tumour models. Precisely how the drug improves oxygenation is not well understood. We hypothesised that metabolic conditions present within solid tumours reduce red blood cell (RBC) deformability and that flunarizine exerts its in vivo effect by preventing this loss of RBC deformability. A microrheometer was used to compare the viscosity of rat and human RBC suspensions in conditions of hypoxia (pO2 < 10 mmHg), acidic environment (pH 6.8), and elevated lactate concentration (lactate 5 mMol l-1), without or with flunarizine at concentrations of 5, 10, and 50 mg l-1. The effects of flunarizine on RBC density and morphology were also recorded. Hypoxia, low pH, and lactate exposure together increased both human and rat RBC suspension viscosity. Flunarizine at concentrations of 5 and 10 mg l-1 prevented the increases in viscosity. The drug caused dose-dependent shifts toward lower cell density while inducing a characteristic cupped shape (stomatcytic morphology), suggesting a mechanism involving calmodulin inhibition. The results support the hypothesis that flunarizine improves tumour blood flow and oxygenation by enhancing flow properties of RBC's in solid tumours.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brereton W., Murphy J. R. Rheologic studies of deoxygenated normal and hereditary spherocytosis blood and separated erythrocytes. J Lab Clin Med. 1974 Jan;83(1):112–118. [PubMed] [Google Scholar]
- Burns N. R., Gratzer W. B. Interaction of calmodulin with the red cell and its membrane skeleton and with spectrin. Biochemistry. 1985 Jun 4;24(12):3070–3074. doi: 10.1021/bi00333a040. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Feo C., Jacobs M. S., Shohet S. B. Separate mechanisms of deformability loss in ATP-depleted and Ca-loaded erythrocytes. J Clin Invest. 1981 Feb;67(2):531–539. doi: 10.1172/JCI110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall E. D., Critz A. M., Osher A. S., Keljo D. J., Forster R. E. Influence of pH on elastic deformability of the human erythrocyte membrane. Am J Physiol. 1978 Nov;235(5):C269–C278. doi: 10.1152/ajpcell.1978.235.5.C269. [DOI] [PubMed] [Google Scholar]
- De Cree J., De Cock W., Geukens H., De Clerck F., Beerens M., Verhaegen H. The rheological effects of cinnarizine and flunarizine in normal and pathologic conditions. Angiology. 1979 Aug;30(8):505–515. doi: 10.1177/000331977903000801. [DOI] [PubMed] [Google Scholar]
- Fenton B. M., Sutherland R. M. Effect of flunarizine on micro-regional distributions of intravascular HbO2 saturations in RIF-1 and KHT sarcomas. Int J Radiat Oncol Biol Phys. 1992;22(3):447–450. doi: 10.1016/0360-3016(92)90850-h. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N., Davis J. I., Foellmer J. W., Casey M. R. Clinical assay of the human erythrocyte lactate transporter. II. Analysis and display of normal human data. Biochem Med Metab Biol. 1988 Jun;39(3):351–359. doi: 10.1016/0885-4505(88)90095-3. [DOI] [PubMed] [Google Scholar]
- Flameng W., Verheyen F., Borgers M., De Clerck F., Brugmans J. The effect of flunarizine treatment on human red blood cells. Angiology. 1979 Aug;30(8):516–525. doi: 10.1177/000331977903000802. [DOI] [PubMed] [Google Scholar]
- Foxdal P., Sjödin B., Rudstam H., Ostman C., Ostman B., Hedenstierna G. C. Lactate concentration differences in plasma, whole blood, capillary finger blood and erythrocytes during submaximal graded exercise in humans. Eur J Appl Physiol Occup Physiol. 1990;61(3-4):218–222. doi: 10.1007/BF00357603. [DOI] [PubMed] [Google Scholar]
- Frank R. S., Hochmuth R. M. The influence of red cell mechanical properties on flow through single capillary-sized pores. J Biomech Eng. 1988 May;110(2):155–160. doi: 10.1115/1.3108421. [DOI] [PubMed] [Google Scholar]
- GULLINO P. M., CLARK S. H., GRANTHAM F. H. THE INTERSTITIAL FLUID OF SOLID TUMORS. Cancer Res. 1964 Jun;24:780–794. [PubMed] [Google Scholar]
- Gross G. P., Hathaway W. E. Fetal erythrocyte deformability. Pediatr Res. 1972 Jul;6(7):593–599. [PubMed] [Google Scholar]
- Guezennec C. Y., Nadaud J. F., Satabin P., Leger F., Lafargue P. Influence of polyunsaturated fatty acid diet on the hemorrheological response to physical exercise in hypoxia. Int J Sports Med. 1989 Aug;10(4):286–291. doi: 10.1055/s-2007-1024917. [DOI] [PubMed] [Google Scholar]
- Hakim T. S., Macek A. S. Effect of hypoxia on erythrocyte deformability in different species. Biorheology. 1988;25(6):857–868. doi: 10.3233/bir-1988-25606. [DOI] [PubMed] [Google Scholar]
- Hermann T., Vasselon C., Geyssant A., Healy J. C. RBC filterability, oxygen saturation, ATP intracellular stock, and cerebral microcirculation. Scand J Clin Lab Invest Suppl. 1981;156:213–216. doi: 10.3109/00365518109097465. [DOI] [PubMed] [Google Scholar]
- Heykants J., De Crée J., Hörig C. Steady-state plasma levels of flunarizine in chronically treated patients. Arzneimittelforschung. 1979;29(8):1168–1171. [PubMed] [Google Scholar]
- Horsman M. R., Chaplin D. J., Overgaard J. The use of blood flow modifiers to improve the treatment response of solid tumors. Radiother Oncol. 1991;20 (Suppl 1):47–52. doi: 10.1016/0167-8140(91)90187-l. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Shrivastav S., Jirtle R. L. Blood flow to primary tumors and lymph node metastases in SMT-2A tumor-bearing rats following intravenous flunarizine. Cancer Res. 1984 Mar;44(3):896–899. [PubMed] [Google Scholar]
- Koutsouris D., Delatour-Hanss E., Hanss M. Physico-chemical factors of erythrocyte deformability. Biorheology. 1985;22(2):119–132. doi: 10.3233/bir-1985-22203. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Follenius A., Gerard D., Stoclet J. C. Bepridil and flunarizine as calmodulin inhibitors. Eur J Pharmacol. 1984 Feb 10;98(1):157–158. doi: 10.1016/0014-2999(84)90128-6. [DOI] [PubMed] [Google Scholar]
- Meiselman H. J. Morphological determinants of red cell deformability. Scand J Clin Lab Invest Suppl. 1981;156:27–34. doi: 10.3109/00365518109097426. [DOI] [PubMed] [Google Scholar]
- Nelson G. A., Andrews M. L., Karnovsky M. J. Control of erythrocyte shape by calmodulin. J Cell Biol. 1983 Mar;96(3):730–735. doi: 10.1083/jcb.96.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Takahashi T., Kon H. A spin-label study of the correlation between stomatocyte formation and membrane fluidization of erythrocytes. Biochem Pharmacol. 1982 Oct 15;31(20):3173–3180. doi: 10.1016/0006-2952(82)90546-9. [DOI] [PubMed] [Google Scholar]
- Noji S., Taniguchi S., Kon H. Spin label study of erythrocyte deformability. Ca2+-induced loss of deformability and the effects of stomatocytogenic reagents on the deformability loss in human erythrocytes in shear flow. Biophys J. 1987 Aug;52(2):221–227. doi: 10.1016/S0006-3495(87)83209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. M., Barker N. D., Rand P. W. Effect of cell geometry, internal viscosity, and pH on erythrocyte filterability. Proc Soc Exp Biol Med. 1981 Mar;166(3):449–456. doi: 10.3181/00379727-166-41089. [DOI] [PubMed] [Google Scholar]
- Rand P. W., Austin W. H., Lacombe E., Barker N. pH and blood viscosity. J Appl Physiol. 1968 Nov;25(5):550–559. doi: 10.1152/jappl.1968.25.5.550. [DOI] [PubMed] [Google Scholar]
- Scheufler E., Vogelgesang R., Wilffert B., Pegram B. L., Hunter J. B., Wermelskirchen D., Peters T. Uptake of catamphiphilic drugs into erythrocytes and muscular tissue correlates to membrane enrichment and to 45Ca displacement from phosphatidylserine monolayers. J Pharmacol Exp Ther. 1990 Jan;252(1):333–338. [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Effect of red blood cell rigidity on tumor blood flow: increase in viscous resistance during hyperglycemia. Cancer Res. 1991 May 15;51(10):2727–2730. [PubMed] [Google Scholar]
- Stone P. H., Antman E. M., Muller J. E., Braunwald E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part II: Hemodynamic effects and clinical applications. Ann Intern Med. 1980 Dec;93(6):886–904. doi: 10.7326/0003-4819-93-6-886. [DOI] [PubMed] [Google Scholar]
- Thomas P. G., Zimmermann A. G., Verkleij A. J. Prevention of calcium-induced membrane structural alterations in erythrocyte membranes by flunarizine. Biochim Biophys Acta. 1988 Dec 22;946(2):439–444. doi: 10.1016/0005-2736(88)90421-x. [DOI] [PubMed] [Google Scholar]
- Tran-Son-Tay R., Coffey B. E., Hochmuth R. M. A rheological study of packed red blood cell suspensions with an oscillating ball microrheometer. Biorheology. 1989;26(2):143–151. doi: 10.3233/bir-1989-26205. [DOI] [PubMed] [Google Scholar]
- Usami S., Chien S., Bertles J. F. Deformability of sickle cells as studied by microsieving. J Lab Clin Med. 1975 Aug;86(2):274–279. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vaupel P., Menke H. Blood flow, vascular resistance and oxygen availability in malignant tumours upon intravenous flunarizine. Adv Exp Med Biol. 1987;215:393–398. doi: 10.1007/978-1-4684-7433-6_48. [DOI] [PubMed] [Google Scholar]
- Waldenlind L., Edlund B. L., Hulting J., Karnell J., Lund F., Rosenhamer G. Decreased red cell filterability in patients with acute myocardial infarction. Acta Med Scand. 1988;224(3):225–229. doi: 10.1111/j.0954-6820.1988.tb19365.x. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Hirst D. G. Cinnarizine and flunarizine as radiation sensitisers in two murine tumours. Br J Cancer. 1988 Dec;58(6):742–745. doi: 10.1038/bjc.1988.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. J., Hirst D. G. Modification of tumour response by calcium antagonists in the SCVII/St tumour implanted at two different sites. Int J Radiat Biol. 1989 Sep;56(3):355–367. doi: 10.1080/09553008914551511. [DOI] [PubMed] [Google Scholar]
- Yardin G., Meiselman H. J. Effects of cellular morphology on the viscoelastic behavior of high hematocrit RBC suspensions. Biorheology. 1989;26(2):153–175. doi: 10.3233/bir-1989-26206. [DOI] [PubMed] [Google Scholar]




