Abstract
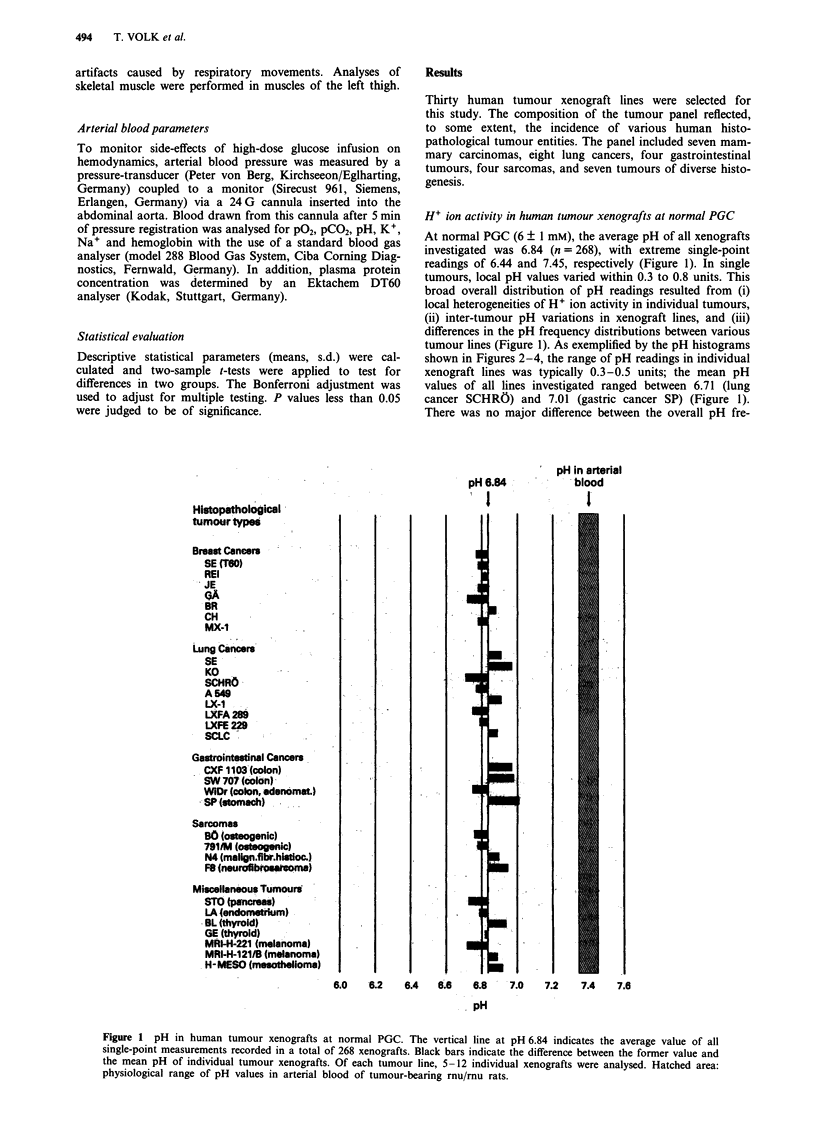
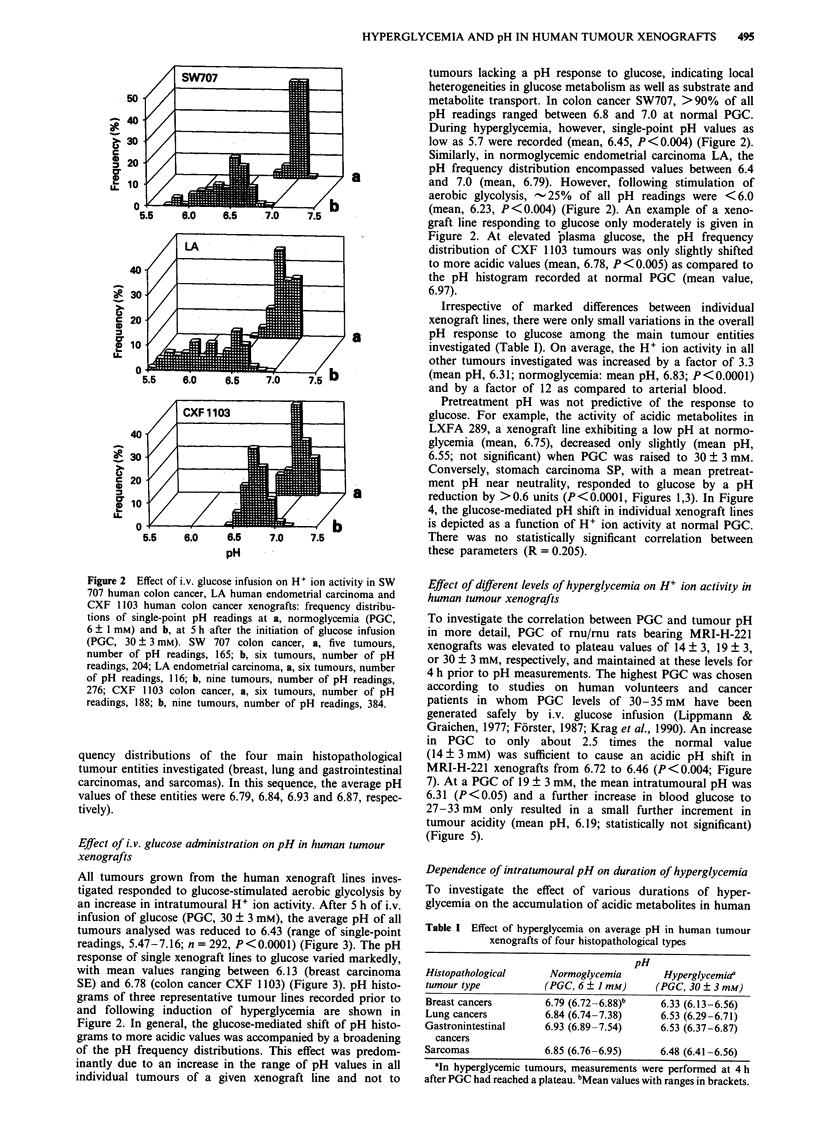
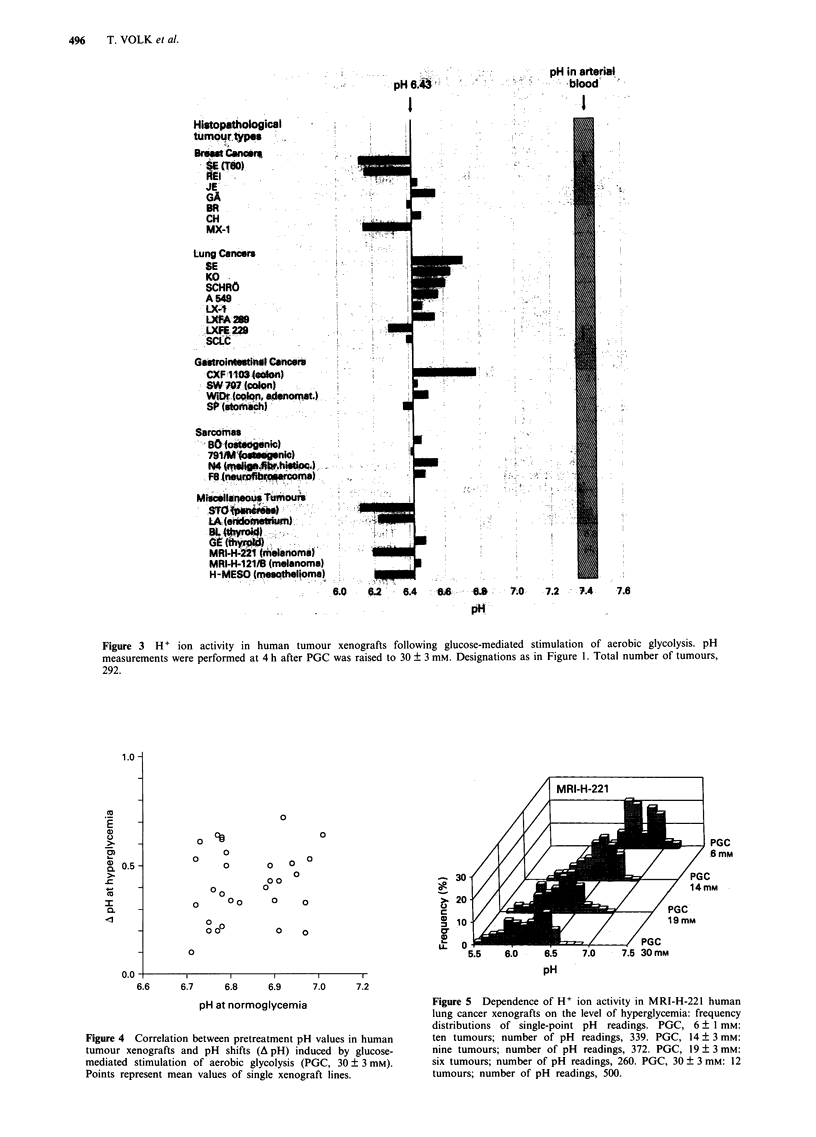
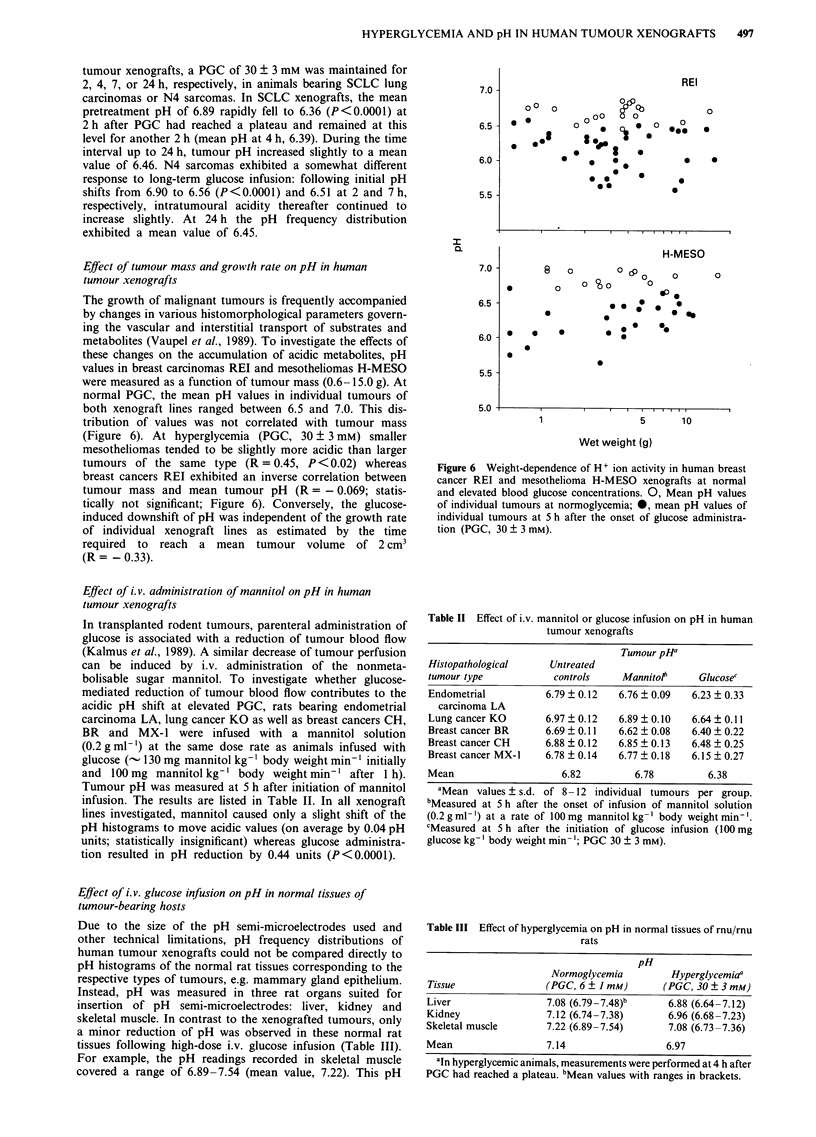
pH frequency distributions of tumours grown s.c. from 30 human tumour xenograft lines in rnu/rnu rats were analysed with the use of H+ ion-sensitive semi-microelectrodes prior to and following stimulation of tumour cell glycolysis by i.v. infusion of glucose. At normoglycemia, the average pH of the tumours investigated was 6.83 (range, 6.72-7.01; n = 268). Without exception, all xenografts responded to the temporary increase in plasma glucose concentration (PGC) from 6 +/- 1 to 30 +/- 3 mM by an accumulation of acidic metabolites, as indicated by a pH reduction to an average value of 6.43 (range, 6.12-6.78; n = 292). This pH value corresponds to a ten-fold increase in H+ ion activity in tumour tissue as compared to arterial blood. Tumour pH approached minimum values at 2-4 h after the onset of glucose administration and could be maintained at acidic levels for 24 h by controlled glucose infusion. Irrespective of pH variations between tumours grown from individual xenograft lines, there was no major difference in pH response to glucose between the four main histopathological tumour entities investigated, i.e. breast, lung and gastrointestinal carcinomas, and sarcomas. In tumours from several xenograft lines, an increase in blood glucose to only 2.5-times the normal value (14 mM) was sufficient to reduce the mean pH to 6.4. Glucose-induced acidosis was tumour-specific. The pH frequency distributions in liver, kidney and skeletal muscle of tumour-bearing rnu/rnu rats were only marginally sensitive to hyperglycemia (average pH, 6.97 vs normal value of 7.14). Tumour-selective activation of pH-sensitive anti-cancer agents, e.g. alkylating drugs, acid-labile prodrugs or pH-sensitive immunoconjugates may thus be feasible in a wide variety of human cancers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby B. S. pH studies in human malignant tumours. Lancet. 1966 Aug 6;2(7458):312–315. doi: 10.1016/s0140-6736(66)92598-0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Busch H. The final common pathway of cancer. Cancer Res. 1990 Aug 15;50(16):4830–4838. [PubMed] [Google Scholar]
- CONNORS T. A., MITCHLEY B. C., ROSENOER V. M., ROSS W. C. THE EFFECT OF GLUCOSE PRETREATMENT ON THE CARCINOSTATIC AND TOXIC ACTIVITIES OF SOME ALKYLATING AGENTS. Biochem Pharmacol. 1964 Mar;13:395–400. doi: 10.1016/0006-2952(64)90158-3. [DOI] [PubMed] [Google Scholar]
- Desmoulin F., Galons J. P., Canioni P., Marvaldi J., Cozzone P. J. 31P nuclear magnetic resonance study of a human colon adenocarcinoma cultured cell line. Cancer Res. 1986 Aug;46(8):3768–3774. [PubMed] [Google Scholar]
- EAGLE H., BARBAN S., LEVY M., SCHULZE H. O. The utilization of carbohydrates by human cell cultures. J Biol Chem. 1958 Sep;233(3):551–558. [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Förster H. Fruktose und Sorbit als energieliefernde Substrate für die parenterale Ernährung. Infusionsther Klin Ernahr. 1987 Jun;14(3):98–109. [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R. Are cancer cells acidic? Br J Cancer. 1991 Sep;64(3):425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Hahn G. M. Comparison between tumor pH and cell sensitivity to heat in RIF-1 tumors. Cancer Res. 1989 Jul 15;49(14):3734–3736. [PubMed] [Google Scholar]
- Hwang Y. C., Kim S. G., Evelhoch J. L., Seyedsadr M., Ackerman J. J. Modulation of murine radiation-induced fibrosarcoma-1 tumor metabolism and blood flow in situ via glucose and mannitol administration monitored by 31P and 2H nuclear magnetic resonance spectroscopy. Cancer Res. 1991 Jun 15;51(12):3108–3118. [PubMed] [Google Scholar]
- Jähde E., Glüsenkamp K. H., Klünder I., Hülser D. F., Tietze L. F., Rajewsky M. F. Hydrogen ion-mediated enhancement of cytotoxicity of bis-chloroethylating drugs in rat mammary carcinoma cells in vitro. Cancer Res. 1989 Jun 1;49(11):2965–2972. [PubMed] [Google Scholar]
- Jähde E., Rajewsky M. F., Baumgärtl H. pH distributions in transplanted neural tumors and normal tissues of BDIX rats as measured with pH microelectrodes. Cancer Res. 1982 Apr;42(4):1498–1504. [PubMed] [Google Scholar]
- Jähde E., Rajewsky M. F. Tumor-selective modification of cellular microenvironment in vivo: effect of glucose infusion on the pH in normal and malignant rat tissues. Cancer Res. 1982 Apr;42(4):1505–1512. [PubMed] [Google Scholar]
- Jähde E., Volk T., Atema A., Smets L. A., Glüsenkamp K. H., Rajewsky M. F. pH in human tumor xenografts and transplanted rat tumors: effect of insulin, inorganic phosphate, and m-iodobenzylguanidine. Cancer Res. 1992 Nov 15;52(22):6209–6215. [PubMed] [Google Scholar]
- Kallinowski F., Schlenger K. H., Runkel S., Kloes M., Stohrer M., Okunieff P., Vaupel P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989 Jul 15;49(14):3759–3764. [PubMed] [Google Scholar]
- Kalmus J., Okunieff P., Vaupel P. Effect of intraperitoneal versus intravenous glucose administration on laser Doppler flow in murine FSaII tumors and normal skin. Cancer Res. 1989 Nov 15;49(22):6313–6317. [PubMed] [Google Scholar]
- Konerding M. A., Steinberg F., Streffer C. The vasculature of xenotransplanted human melanomas and sarcomas on nude mice. I. Vascular corrosion casting studies. Acta Anat (Basel) 1989;136(1):21–26. doi: 10.1159/000146792. [DOI] [PubMed] [Google Scholar]
- Konerding M. A., Steinberg F., Streffer C. The vasculature of xenotransplanted human melanomas and sarcomas on nude mice. II. Scanning and transmission electron microscopic studies. Acta Anat (Basel) 1989;136(1):27–33. doi: 10.1159/000146793. [DOI] [PubMed] [Google Scholar]
- Krag D. N., Storm F. K., Morton D. L. Induction of transient hyperglycaemia in cancer patients. Int J Hyperthermia. 1990 Jul-Aug;6(4):741–744. doi: 10.3109/02656739009140821. [DOI] [PubMed] [Google Scholar]
- Krüger W., Mayer W. K., Schaefer C., Stohrer M., Vaupel P. Acute changes of systemic parameters in tumour-bearing rats, and of tumour glucose, lactate, and ATP levels upon local hyperthermia and/or hyperglycaemia. J Cancer Res Clin Oncol. 1991;117(5):409–415. doi: 10.1007/BF01612759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie E., Hirschberg D. L., Schreiber G., Thor K., Hill L., Hellstrom I., Hellstrom K. E. Monoclonal antibody L6-daunomycin conjugates constructed to release free drug at the lower pH of tumor tissue. Cancer Immunol Immunother. 1991;33(4):223–230. doi: 10.1007/BF01744941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppmann H. G., Graichen D. Glukose- und K+-Bilanz während hochdosierter intravenöser Glukosezufuhr. Infusionsther Klin Ernahr. 1977 Jun;4(3):166–178. [PubMed] [Google Scholar]
- Lyon R. C., Cohen J. S., Faustino P. J., Megnin F., Myers C. E. Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res. 1988 Feb 15;48(4):870–877. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- NAESLUND J., SWENSON K. E. Investigations on the pH of malignant tumors in mice and humans after the administration of glucose. Acta Obstet Gynecol Scand. 1953;32(3):359–367. doi: 10.3109/00016345309157588. [DOI] [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Thistlethwaite A. J., Alexander G. A., Moylan D. J., 3rd, Leeper D. B. Modification of human tumor pH by elevation of blood glucose. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):603–610. doi: 10.1016/0360-3016(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Tietze L. F., Neumann M., Möllers T., Fischer R., Glüsenkamp K. H., Rajewsky M. F., Jähde E. Proton-mediated liberation of aldophosphamide from a nontoxic prodrug: a strategy for tumor-selective activation of cytocidal drugs. Cancer Res. 1989 Aug 1;49(15):4179–4184. [PubMed] [Google Scholar]
- Vaupel P., Fortmeyer H. P., Runkel S., Kallinowski F. Blood flow, oxygen consumption, and tissue oxygenation of human breast cancer xenografts in nude rats. Cancer Res. 1987 Jul 1;47(13):3496–3503. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Volk T., Roszinski S., Jähde E., Glüsenkamp K. H., Rajewsky M. F. Effect of glucose-mediated pH reduction and cyclophosphamide on oxygenation of transplanted rat tumors. Int J Radiat Oncol Biol Phys. 1993 Feb 15;25(3):465–471. doi: 10.1016/0360-3016(93)90068-7. [DOI] [PubMed] [Google Scholar]
- Ward K. A., Jain R. K. Response of tumours to hyperglycaemia: characterization, significance and role in hyperthermia. Int J Hyperthermia. 1988 May-Jun;4(3):223–250. doi: 10.3109/02656738809051100. [DOI] [PubMed] [Google Scholar]
- Weber G. Enzymology of cancer cells (second of two parts). N Engl J Med. 1977 Mar 10;296(10):541–551. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Seino Y., Fukumoto H., Koh G., Yano H., Inagaki N., Yamada Y., Inoue K., Manabe T., Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990 Jul 16;170(1):223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- Yatvin M. B., Kreutz W., Horwitz B. A., Shinitzky M. pH-sensitive liposomes: possible clinical implications. Science. 1980 Dec 12;210(4475):1253–1255. doi: 10.1126/science.7434025. [DOI] [PubMed] [Google Scholar]
- von Ardenne M., Reitnauer P. G. Uber manipulierte Ubersäuerung autochthoner Tumoren. Onkologie. 1978 Apr;1(2):85–88. doi: 10.1159/000213922. [DOI] [PubMed] [Google Scholar]



