Abstract
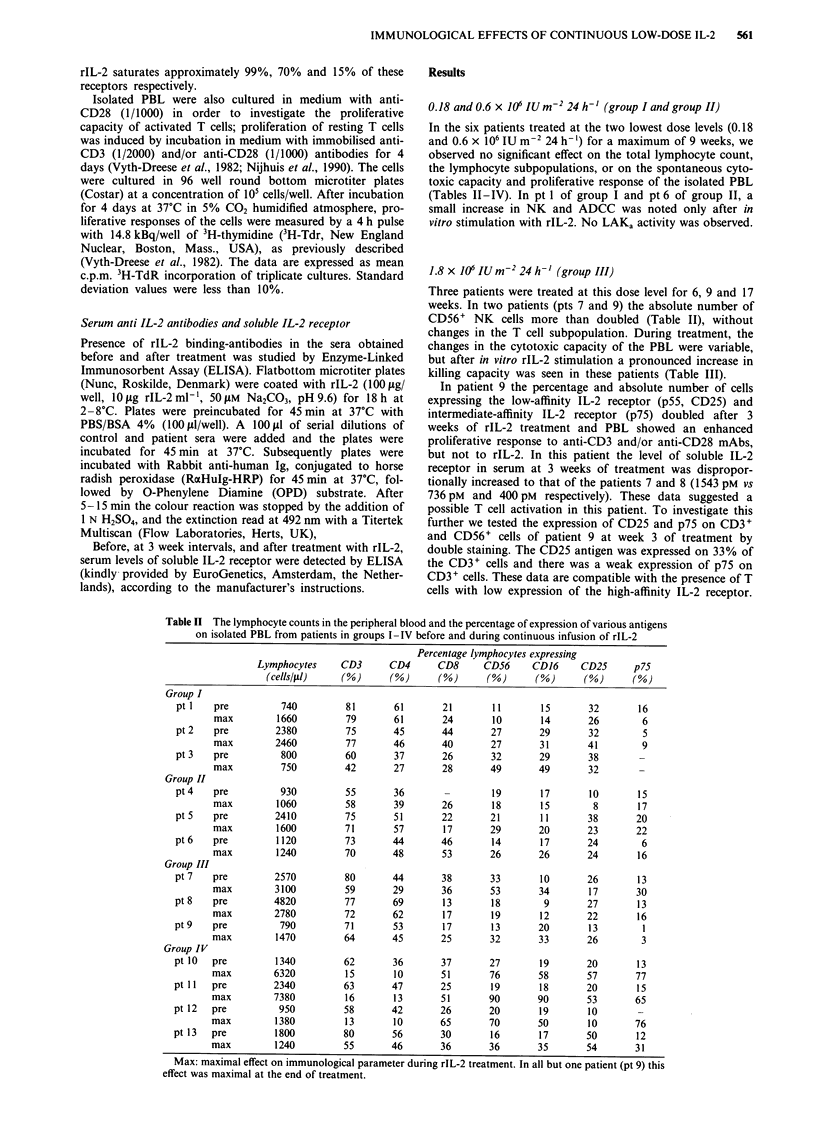
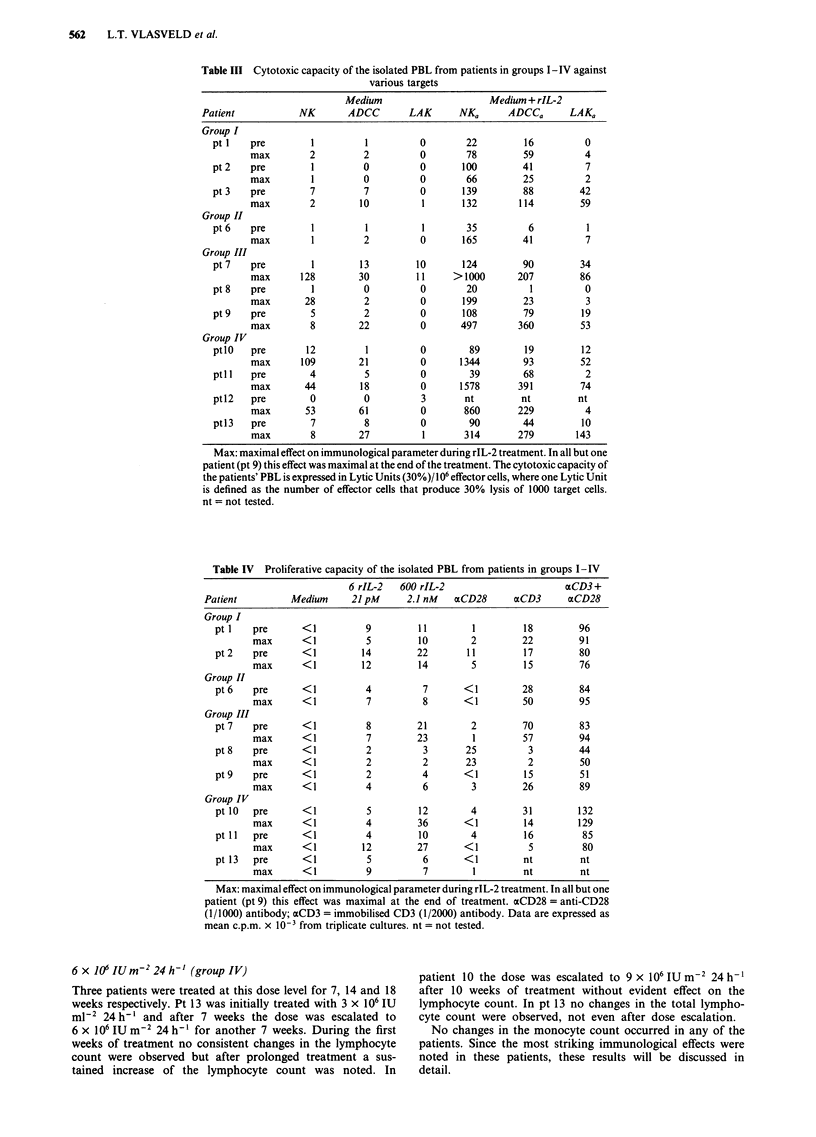
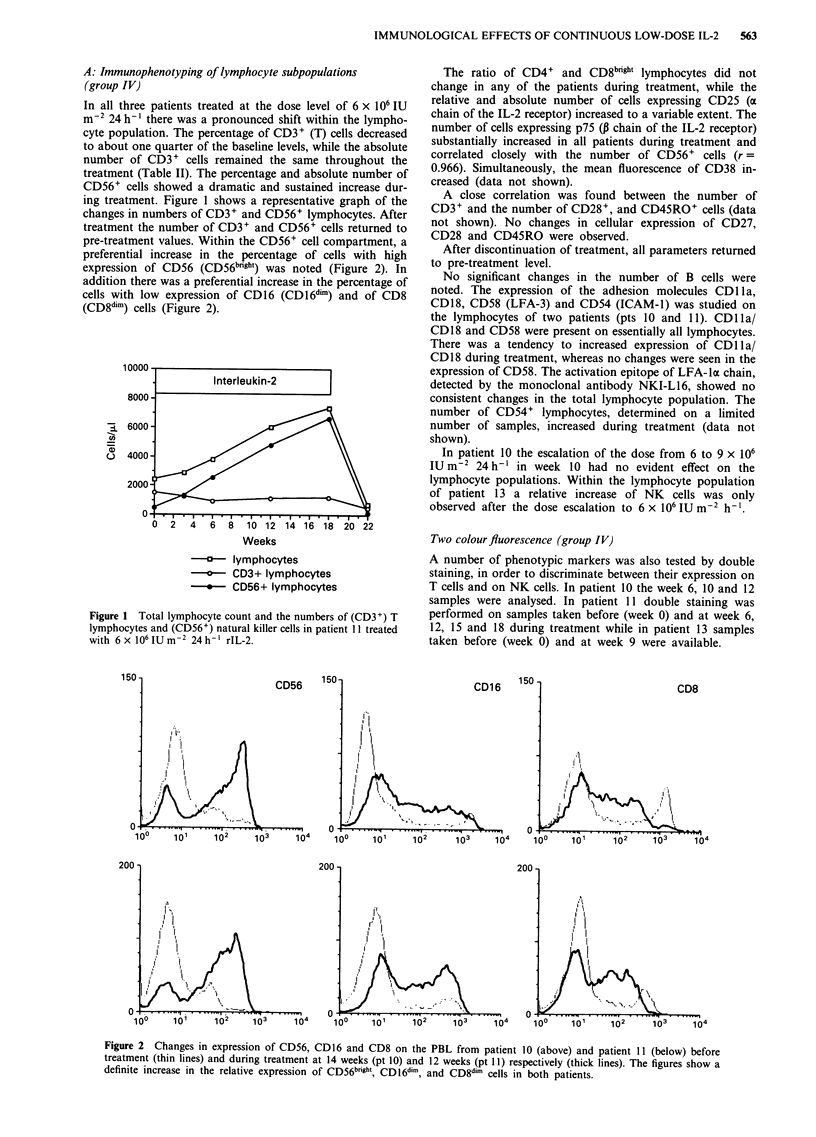
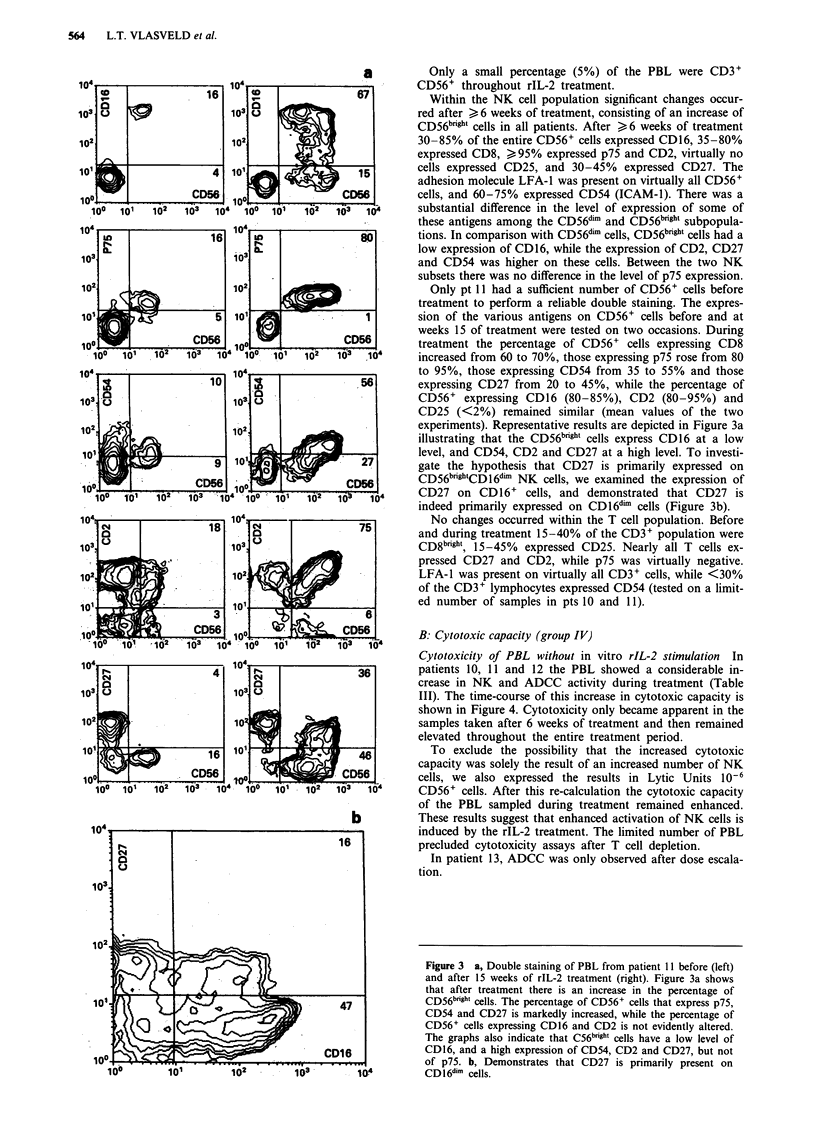
Previously we described the clinical aspects of a phase I study of prolonged continuous infusion of low-dose recombinant interleukin-2 (rIL-2). In the present paper we report several immunological effects in 13 patients with melanoma and renal cell cancer treated on an out-patient basis with rIL-2 for uninterrupted periods ranging from 5 to 18 weeks. Groups of three patients were treated at following dose levels 0.18, 0.6, 1.8 or 6 x 10(6) IU m-2 24 h-1 and one patient was treated with 3 x 10(6) IU m-2 24 h-1. Prolonged rIL-2 treatment resulted in a dose-dependent and sustained increase in the percentage and absolute number of (CD56+, CD8dim) natural killer cells. Within this population a preferential increase in the CD56bright cells with low expression of CD16 was observed. The CD27 antigen was also upregulated in the CD56bright CD16dim population. This increase of NK cells was accompanied by an enhancement of the cytotoxic capacity of the peripheral lymphocytes. No consistent signs of T cell activation or expansion were noted.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogner M. P., Voss S. D., Bechhofer R., Hank J. A., Roper M., Poplack D., Hammond D., Sondel P. M. Serum CD25 levels during interleukin-2 therapy: dose dependence and correlations with clinical toxicity and lymphocyte surface sCD25 expression. J Immunother (1991) 1992 Feb;11(2):111–118. [PubMed] [Google Scholar]
- Caligiuri M. A., Murray C., Soiffer R. J., Klumpp T. R., Seiden M., Cochran K., Cameron C., Ish C., Buchanan L., Perillo D. Extended continuous infusion low-dose recombinant interleukin-2 in advanced cancer: prolonged immunomodulation without significant toxicity. J Clin Oncol. 1991 Dec;9(12):2110–2119. doi: 10.1200/JCO.1991.9.12.2110. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creekmore S. P., Harris J. E., Ellis T. M., Braun D. P., Cohen I. I., Bhoopalam N., Jassak P. F., Cahill M. A., Canzoneri C. L., Fisher R. I. A phase I clinical trial of recombinant interleukin-2 by periodic 24-hour intravenous infusions. J Clin Oncol. 1989 Feb;7(2):276–284. doi: 10.1200/JCO.1989.7.2.276. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., Creekmore S. P., McMannis J. D., Braun D. P., Harris J. A., Fisher R. I. Appearance and phenotypic characterization of circulating Leu 19+ cells in cancer patients receiving recombinant interleukin 2. Cancer Res. 1988 Nov 15;48(22):6597–6602. [PubMed] [Google Scholar]
- Ellis T. M., Fisher R. I. Functional heterogeneity of Leu 19"bright"+ and Leu 19"dim"+ lymphokine-activated killer cells. J Immunol. 1989 Apr 15;142(8):2949–2954. [PubMed] [Google Scholar]
- Gambacorti-Passerini C., Radrizzani M., Marolda R., Belli F., Sciorelli G., Galazka A. R., Schindler J. D., Cascinelli N., Parmiani G. In vivo activation of lymphocytes in melanoma patients receiving escalating doses of recombinant interleukin 2. Int J Cancer. 1988 May 15;41(5):700–706. doi: 10.1002/ijc.2910410511. [DOI] [PubMed] [Google Scholar]
- Gemlo B. T., Palladino M. A., Jr, Jaffe H. S., Espevik T. P., Rayner A. A. Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Oct 15;48(20):5864–5867. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hank J. A., Kohler P. C., Weil-Hillman G., Rosenthal N., Moore K. H., Storer B., Minkoff D., Bradshaw J., Bechhofer R., Sondel P. M. In vivo induction of the lymphokine-activated killer phenomenon: interleukin 2-dependent human non-major histocompatibility complex-restricted cytotoxicity generated in vivo during administration of human recombinant interleukin 2. Cancer Res. 1988 Apr 1;48(7):1965–1971. [PubMed] [Google Scholar]
- Hank J. A., Robinson R. R., Surfus J., Mueller B. M., Reisfeld R. A., Cheung N. K., Sondel P. M. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990 Sep 1;50(17):5234–5239. [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Hofmann B., Bass H., Nishanian P., Faisal M., Figlin R. A., Sarna G. P., Fahey J. L. Different lymphoid cell populations produce varied levels of neopterin, beta 2-microglobulin and soluble IL-2 receptor when stimulated with IL-2, interferon-gamma or tumour necrosis factor-alpha. Clin Exp Immunol. 1992 Jun;88(3):548–554. doi: 10.1111/j.1365-2249.1992.tb06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. C., Hank J. A., Moore K. H., Storer B., Bechhofer R., Hong R., Sondel P. M. Phase 1 clinical trial of recombinant interleukin-2: a comparison of bolus and continuous intravenous infusion. Cancer Invest. 1989;7(3):213–223. doi: 10.3109/07357908909039840. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Liang S. M., Allet B., Rose K., Hirschi M., Liang C. M., Thatcher D. R. Characterization of human interleukin 2 derived from Escherichia coli. Biochem J. 1985 Jul 15;229(2):429–439. doi: 10.1042/bj2290429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissoni P., Tisi E., Brivio F., Barni S., Rovelli F., Perego M., Tancini G. Increase in soluble interleukin-2 receptor and neopterin serum levels during immunotherapy of cancer with interleukin-2. Eur J Cancer. 1991;27(8):1014–1016. doi: 10.1016/0277-5379(91)90271-e. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Custer M. C., Sharrow S. O., Rubin L. A., Nelson D. L., Rosenberg S. A. In vivo administration of purified human interleukin-2 to patients with cancer: development of interleukin-2 receptor positive cells and circulating soluble interleukin-2 receptors following interleukin-2 administration. Cancer Res. 1987 Apr 15;47(8):2188–2195. [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Phillips J. H. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990 May 1;171(5):1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhuis E. W., vd Wiel-van Kemenade E., Figdor C. G., van Lier R. A. Activation and expansion of tumour-infiltrating lymphocytes by anti-CD3 and anti-CD28 monoclonal antibodies. Cancer Immunol Immunother. 1990;32(4):245–250. doi: 10.1007/BF01741708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Robertson M. J., Caligiuri M. A., Manley T. J., Levine H., Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- Robertson M. J., Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990 Dec 15;76(12):2421–2438. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Nelson D. L. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med. 1990 Oct 15;113(8):619–627. doi: 10.7326/0003-4819-113-8-619. [DOI] [PubMed] [Google Scholar]
- Schaafsma M. R., Falkenburg J. H., Landegent J. E., Duinkerken N., Osanto S., Ralph P., Kaushansky K., Wagemaker G., Van Damme J., Willemze R. In vivo production of interleukin-5, granulocyte-macrophage colony-stimulating factor, macrophages colony-stimulating factor, and interleukin-6 during intravenous administration of high-dose interleukin-2 in cancer patients. Blood. 1991 Oct 15;78(8):1981–1987. [PubMed] [Google Scholar]
- Soiffer R. J., Murray C., Cochran K., Cameron C., Wang E., Schow P. W., Daley J. F., Ritz J. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992 Jan 15;79(2):517–526. [PubMed] [Google Scholar]
- Sondel P. M., Kohler P. C., Hank J. A., Moore K. H., Rosenthal N. S., Sosman J. A., Bechhofer R., Storer B. Clinical and immunological effects of recombinant interleukin 2 given by repetitive weekly cycles to patients with cancer. Cancer Res. 1988 May 1;48(9):2561–2567. [PubMed] [Google Scholar]
- Sugita K., Robertson M. J., Torimoto Y., Ritz J., Schlossman S. F., Morimoto C. Participation of the CD27 antigen in the regulation of IL-2-activated human natural killer cells. J Immunol. 1992 Aug 15;149(4):1199–1203. [PubMed] [Google Scholar]
- Sugita K., Torimoto Y., Nojima Y., Daley J. F., Schlossman S. F., Morimoto C. The 1A4 molecule (CD27) is involved in T cell activation. J Immunol. 1991 Sep 1;147(5):1477–1483. [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Lee D. J., Lindgren C. G., Benz L. A., Collins C., Levitt D., Fefer A. Influence of dose and duration of infusion of interleukin-2 on toxicity and immunomodulation. J Clin Oncol. 1988 Apr;6(4):669–678. doi: 10.1200/JCO.1988.6.4.669. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Lee D. J., Lindgren C. G., Benz L. A., Collins C., Shuman W. P., Levitt D., Fefer A. Influence of schedule of interleukin 2 administration on therapy with interleukin 2 and lymphokine activated killer cells. Cancer Res. 1989 Jan 1;49(1):235–240. [PubMed] [Google Scholar]
- Triozzi P. L., Eicher D. M., Rinehart J. J. Modulation of adhesion molecules on human large granular lymphocytes by interleukin-2 in vivo and in vitro. Cell Immunol. 1992 Apr;140(2):295–303. doi: 10.1016/0008-8749(92)90197-w. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Kitamura F., Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1982–1986. doi: 10.1073/pnas.86.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urba W. J., Steis R. G., Longo D. L., Kopp W. C., Maluish A. E., Marcon L., Nelson D. L., Stevenson H. C., Clark J. W. Immunomodulatory properties and toxicity of interleukin 2 in patients with cancer. Cancer Res. 1990 Jan 1;50(1):185–192. [PubMed] [Google Scholar]
- Vlasveld L. T., Rankin E. M., Hekman A., Rodenhuis S., Beijnen J. H., Hilton A. M., Dubbelman A. C., Vyth-Dreese F. A., Melief C. J. A phase I study of prolonged continuous infusion of low dose recombinant interleukin-2 in melanoma and renal cell cancer. Part I: Clinical aspects. Br J Cancer. 1992 May;65(5):744–750. doi: 10.1038/bjc.1992.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Hank J. A., Nobis C. A., Fisch P., Sosman J. A., Sondel P. M. Serum levels of the low-affinity interleukin-2 receptor molecule (TAC) during IL-2 therapy reflect systemic lymphoid mass activation. Cancer Immunol Immunother. 1989;29(4):261–269. doi: 10.1007/BF00199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Robb R. J., Weil-Hillman G., Hank J. A., Sugamura K., Tsudo M., Sondel P. M. Increased expression of the interleukin 2 (IL-2) receptor beta chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J Exp Med. 1990 Oct 1;172(4):1101–1114. doi: 10.1084/jem.172.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyth-Dreese F. A., van der Reijden H. J., de Vries J. E. Phorbol-ester-mediated induction and augmentation of mitogenesis and interleukin-2 production in human T-cell lymphoproliferative disease. Blood. 1982 Dec;60(6):1437–1446. [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Hillman G., Fisch P., Prieve A. F., Sosman J. A., Hank J. A., Sondel P. M. Lymphokine-activated killer activity induced by in vivo interleukin 2 therapy: predominant role for lymphocytes with increased expression of CD2 and leu19 antigens but negative expression of CD16 antigens. Cancer Res. 1989 Jul 1;49(13):3680–3688. [PubMed] [Google Scholar]
- Weil-Hillman G., Voss S. D., Fisch P., Schell K., Hank J. A., Sosman J. A., Sugamura K., Sondel P. M. Natural killer cells activated by interleukin 2 treatment in vivo respond to interleukin 2 primarily through the p75 receptor and maintain the p55 (TAC) negative phenotype. Cancer Res. 1990 May 1;50(9):2683–2691. [PubMed] [Google Scholar]
- Yoshino I., Yano T., Murata M., Ishida T., Sugimachi K., Kimura G., Nomoto K. Cytolytic potential of peripheral blood T-lymphocytes following adoptive immunotherapy with lymphokine-activated killer cells and low-dose interleukin 2. Cancer Res. 1991 Mar 1;51(5):1494–1498. [PubMed] [Google Scholar]
- van Lier R. A., Borst J., Vroom T. M., Klein H., Van Mourik P., Zeijlemaker W. P., Melief C. J. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987 Sep 1;139(5):1589–1596. [PubMed] [Google Scholar]



