Abstract
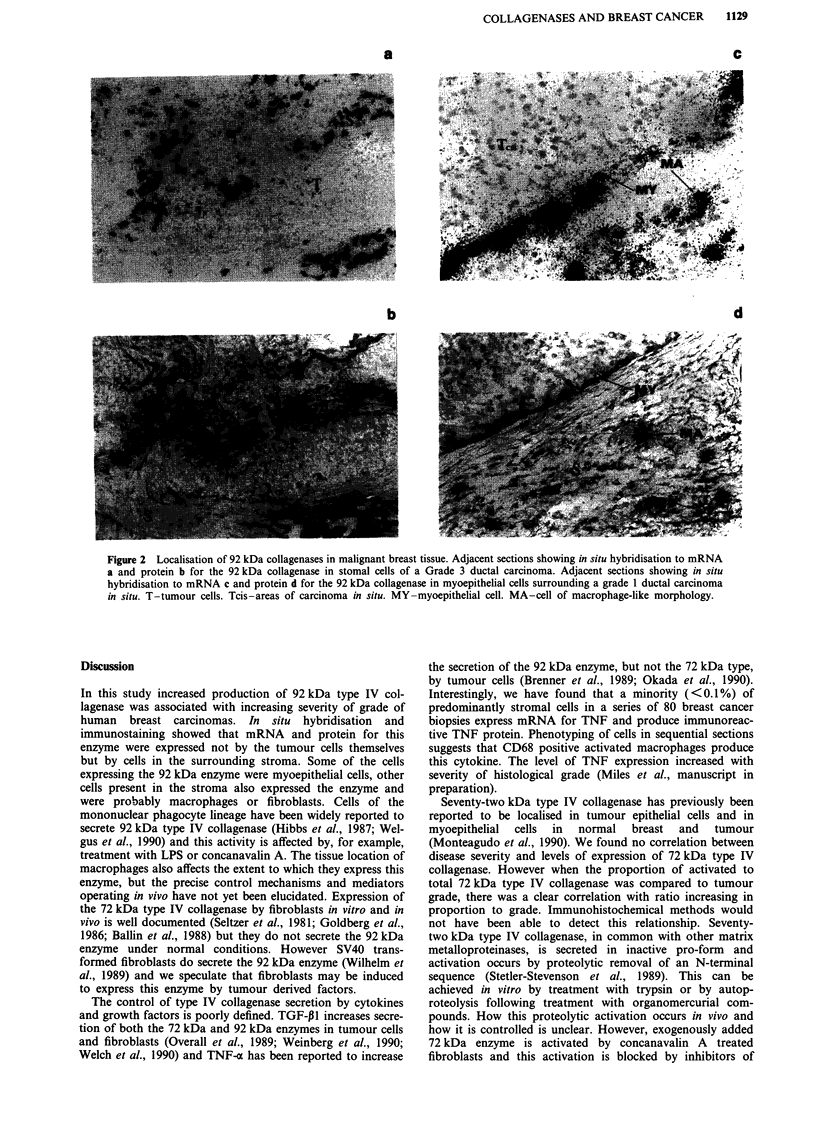
Using zymography and computer assisted image analysis, we have measured the levels of type IV collagenases in biopsies from normal breast, and benign and malignant breast disease. The 92 kDa form was present in three of 11 cases of normal/benign disease, three of nine grade I tumours, four of 12 grade II tumours, but 11 of 11 grade III tumours. Mean levels were higher in grade III tumours (P < 0.0001). When the levels of 72 kDa collagenase and its active 62 kDa form were considered together, there was no difference between the benign and malignant cases (P = 0.55), but the amount of active enzyme, considered as a proportion of the 62 + 72 kDa forms, was significantly higher in malignant disease (P = 0.003). There was also a trend towards a higher proportion of active enzyme with increasing tumour grade (P < 0.0001). In situ hybridisation and immunohistochemistry studies showed that that mRNA and protein for the 92 kDa enzyme was primarily found in the tumour stroma. mRNA for the 72 kDa enzyme was also found in stromal areas. This study demonstrates a clear relationship between production of Type IV collagenases and malignant breast disease. Inhibitors of these enzymes may be of value in preventing metastatic disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O. A., Carmichael D. F., DeClerck Y. A. Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. J Natl Cancer Inst. 1990 Apr 4;82(7):589–595. doi: 10.1093/jnci/82.7.589. [DOI] [PubMed] [Google Scholar]
- Ballin M., Gomez D. E., Sinha C. C., Thorgeirsson U. P. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988 Aug 15;154(3):832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Daidone M. G., Silvestrini R., D'Errico A., Di Fronzo G., Benini E., Mancini A. M., Garbisa S., Liotta L. A., Grigioni W. F. Laminin receptors, collagenase IV and prognosis in node-negative breast cancers. Int J Cancer. 1991 Jun 19;48(4):529–532. doi: 10.1002/ijc.2910480409. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A., Perez N., Shimada H., Boone T. C., Langley K. E., Taylor S. M. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992 Feb 1;52(3):701–708. [PubMed] [Google Scholar]
- Elston C. W. The assessment of histological differentiation in breast cancer. Aust N Z J Surg. 1984 Feb;54(1):11–15. doi: 10.1111/j.1445-2197.1984.tb06677.x. [DOI] [PubMed] [Google Scholar]
- Garbisa S., Negro A., Kalebic T., Pozzatti R., Muschel R., Saffiotti U., Liotta L. A. Type IV collagenolytic activity linkage with the metastatic phenotype induced by ras transfection. Adv Exp Med Biol. 1988;233:179–186. doi: 10.1007/978-1-4899-5037-6_20. [DOI] [PubMed] [Google Scholar]
- Garbisa S., Pozzatti R., Muschel R. J., Saffiotti U., Ballin M., Goldfarb R. H., Khoury G., Liotta L. A. Secretion of type IV collagenolytic protease and metastatic phenotype: induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-E1a. Cancer Res. 1987 Mar 15;47(6):1523–1528. [PubMed] [Google Scholar]
- Goldberg G. I., Frisch S. M., He C., Wilhelm S. M., Reich R., Collier I. E. Secreted proteases. Regulation of their activity and their possible role in metastasis. Ann N Y Acad Sci. 1990;580:375–384. doi: 10.1111/j.1749-6632.1990.tb17945.x. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. T., Cioce V., Sobel M. E., Garbisa S., Grigioni W. F., Liotta L. A., Stetler-Stevenson W. G. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res. 1991 Jan 1;51(1):439–444. [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991 Sep 15;51(18 Suppl):5054s–5059s. [PubMed] [Google Scholar]
- Monteagudo C., Merino M. J., San-Juan J., Liotta L. A., Stetler-Stevenson W. G. Immunohistochemical distribution of type IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol. 1990 Mar;136(3):585–592. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Balkwill F. R. Investigation of cytokine gene expression in human colorectal cancer. Cancer Res. 1990 Jul 15;50(14):4436–4440. [PubMed] [Google Scholar]
- Okada Y., Tsuchiya H., Shimizu H., Tomita K., Nakanishi I., Sato H., Seiki M., Yamashita K., Hayakawa T. Induction and stimulation of 92-kDa gelatinase/type IV collagenase production in osteosarcoma and fibrosarcoma cell lines by tumor necrosis factor alpha. Biochem Biophys Res Commun. 1990 Sep 14;171(2):610–617. doi: 10.1016/0006-291x(90)91190-4. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Huhtala P., Hurskainen T., Danø K., Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992 Mar 1;52(5):1336–1341. [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Siegal G. P., Barsky S. H., Terranova V. P., Liotta L. A. Stages of neoplastic transformation of human breast tissue as monitored by dissolution of basement membrane components. An immunoperoxidase study. Invasion Metastasis. 1981;1(1):54–70. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Ward R. V., Atkinson S. J., Slocombe P. M., Docherty A. J., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases-2 inhibits the activation of 72 kDa progelatinase by fibroblast membranes. Biochim Biophys Acta. 1991 Aug 30;1079(2):242–246. doi: 10.1016/0167-4838(91)90132-j. [DOI] [PubMed] [Google Scholar]
- Weinberg W. C., Brown P. D., Stetler-Stevenson W. G., Yuspa S. H. Growth factors specifically alter hair follicle cell proliferation and collagenolytic activity alone or in combination. Differentiation. 1990 Dec;45(3):168–178. doi: 10.1111/j.1432-0436.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Yagel S., Warner A. H., Nellans H. N., Lala P. K., Waghorne C., Denhardt D. T. Suppression by cathepsin L inhibitors of the invasion of amnion membranes by murine cancer cells. Cancer Res. 1989 Jul 1;49(13):3553–3557. [PubMed] [Google Scholar]